Page 26 - IJB-2-1
P. 26
Preventing bacterial adhesion on scaffolds for bone tissue engineering
(1) Zwitterionization of metal substrates ated atom transfer radical polymerization (SI-ATRP) [33] .
Zwiterionic polymers such poly(sulfobetaine metha- Among zwitterionic polymers, pSBMA has been gra-
crylate) (pSBMA) and poly(carboxybetaine metha- fted to different substrates such as gold [33] , glass [34]
crylate) (pCBMA), possessing mixed positively and and poly(tetrafluoroethylene) membranes [35] to attain
negatively charged functional groups within the same unfouling surfaces.
polymer chain and total neutral charge, exhibit ultra- Recently, an improved strategy for surface zwitte-
low fouling capabilities, able to inhibit nonspecific rionization of metallic surfaces, such as commercial
protein adsorption, bacterial adhesion and biofilm pure titanium (pTi) [36] and biomedical grade 316L type
. The most widely used method to graft
formation [28–32] stainless steel (SUS 316L) [37] , by SI-ATRP of pSBMA
zwitterionic polymers to surfaces is the surface-initi- has been reported (Figure 1A). Zwitterionization can
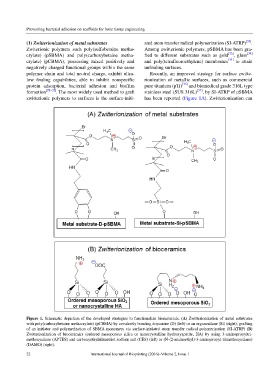
Figure 1. Schematic depiction of the developed strategies to functionalize biomaterials. (A) Zwitterionization of metal substrates
with poly(carboxybetaine methacrylate) (pCBMA) by covalently bonding dopamine (D) (left) or an organosilane (Si) (right), grafting
of an initiator and polymerization of SBMA monomers via surface-initiated atom transfer radical polymerization (SI-ATRP) (B)
Zwitterionization of bioceramics (ordered mesoporous silica or nanocrystalline hydroxyapatite, HA) by using 3-aminopropyltri-
methoxysilane (APTES) and carboxyethylsilanetriol sodium salt (CES) (left) or (N-(2-aminoethyl)-3-aminopropyl-trimethoxysilane)
(DAMO) (right).
22 International Journal of Bioprinting (2016)–Volume 2, Issue 1