Page 45 - IJB-2-1
P. 45
Christopher Chi Wai Tse, Shea Shin Ng, Jonathan Stringer, et al.
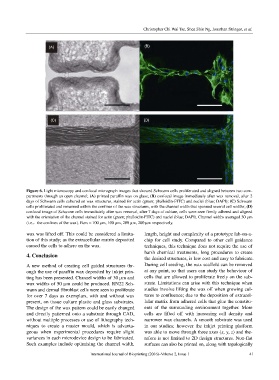
Figure 6. Light microscopy and confocal micrograph images that showed Schwann cells proliferated and aligned between two com-
partments through an open channel; (A) printed paraffin wax on glass; (B) confocal image immediately after wax removal, after 2
days of Schwann cells cultured on wax structures, stained for actin (green; phalloidin-FITC) and nuclei (blue; DAPI); (C) Schwann
cells proliferated and remained within the confines of the wax structures, with the channel width that spanned several cell widths; (D)
confocal image of Schwann cells immediately after wax removal, after 7 days of culture, cells were seen firmly adhered and aligned
with the orientation of the channel stained for actin (green; phalloidin-FITC) and nuclei (blue; DAPI). Channel widths averaged 30 µm
(i.e.,the confines of the wax). Bars = 100 µm, 100 µm, 200 µm, 200 µm respectively.
wax was lifted off. This could be considered a limita- length, height and complexity of a prototype lab-on-a-
tion of this study; as the extracellular matrix deposited chip for cell study. Compared to other cell guidance
caused the cells to adhere on the wax. techniques, this technique does not require the use of
harsh chemical treatments, long procedures to create
4. Conclusion
the desired structures, is low cost and easy to fabricate.
A new method of creating cell guided structures thr- During cell seeding, the wax scaffold can be removed
ough the use of paraffin wax deposited by inkjet prin- at any point, so that users can study the behaviour of
ting has been presented. Channel widths of 30 µm and cells that are allowed to proliferate freely on the sub-
wax widths of 50 µm could be produced. RN22 Sch- strate. Limitations can arise with this technique when
wann and dermal fibroblast cells were seen to proliferate studies involve lifting the wax off when growing cul-
for over 7 days as exemplars, with and without wax tures to confluence; due to the deposition of extracel-
present, on tissue culture plastic and glass substrates. lular matrix from adhered cells that glue the constitu-
The design of the wax pattern could be easily changed ents of the surrounding environment together. More
and directly patterned onto a substrate through CAD, cells are lifted off with increasing cell density and
without multiple processes or use of lithography tech- narrower wax channels. A smooth substrate was used
niques to create a master mould, which is advanta- in our studies; however the inkjet printing platform
geous when experimental procedures require slight was able to move through three axes (x, y, z) and the-
variances in each microdevice design to be fabricated. refore is not limited to 2D design structures. Non-flat
Such examples include optimising the channel width, surfaces can also be printed on, along with topologically
International Journal of Bioprinting (2016)–Volume 2, Issue 1 41