Page 104 - IJB-10-2
P. 104
International Journal of Bioprinting 3D-printed nanocomposites: Synthesis & applications
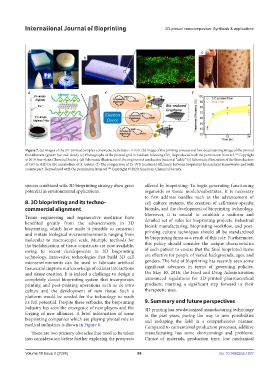
Figure 7. (a) Images of the 3D-printed complex constructs. Scale bars = 5 mm. (b) Image of the printing process and live-dead staining image of the printed
178
thin filament (green: live; red: dead). (c) Photographs of the printed grid in medium releasing CO . Reproduced with the permission from ref. Copyright
2
© 2019 American Chemical Society. (d) Schematic illustration of the engineered conductive bacterial “cable.” (e) Schematic illustration of the bioreduction
of GO to rGO by the metabolism of S. loihica. (f) The comparison of Cr (VI) treatment efficiency between bioprinted hierarchical frameworks and bulk
182
counterpart. Reproduced with the permission from ref. Copyright © 2020 American Chemical Society.
species combined with 3D bioprinting strategy show great offered by bioprinting. To begin generating functioning
potential in environmental applications. organoids or tissue models/substitutes, it is necessary
to first address hurdles such as the advancement of
8. 3D bioprinting and its techno- cell culture systems, the creation of cell/tissue-specific
commercial alignment bioinks, and the development of bioprinting technology.
Moreover, it is crucial to establish a uniform and
Tissue engineering and regenerative medicine have detailed set of rules for bioprinting projects. Industrial
benefited greatly from the advancements in 3D
bioprinting, which have made it possible to construct bioink manufacturing, bioprinting workflow, and post-
and imitate biological microenvironments ranging from printing culture techniques should all be standardized
molecular to macroscopic scale. Multiple methods for by bioprinting firms as a result of this rule. Furthermore,
the biofabrication of tissue constructs are now available, this policy should consider the unique characteristics
owing to recent developments in 3D bioprinting of each patient to ensure that the final bioprinted items
technology. Innovative technologies that build 3D cell are effective for people of varied backgrounds, ages, and
microenvironments can be used to fabricate artificial genders. The field of bioprinting has recently seen some
tissues and improve our knowledge of cellular interactions significant advances in terms of governing policies.
and tissue creation. It is indeed a challenge to design a On May 10, 2016, the Food and Drug Administration
completely closed bioprinting system that incorporates announced regulations for 3D-printed pharmaceutical
printing and post-printing operations such as in vitro products, marking a significant step forward in their
culture and the development of new tissue. Such a therapeutic uses.
platform would be needed for the technology to reach
its full potential. Despite these setbacks, the bioprinting 9. Summary and future perspectives
industry has seen the emergence of new players and the 3D printing has revolutionized manufacturing technology
forging of new alliances. A brief information of some in the past years, paving the way to new possibilities
bioprinting companies which are playing pivotal role in and reshaping the field in a comprehensive manner.
medical industries is shown in Figure 8. Compared to conventional production processes, additive
There are two primary obstacles that need to be taken manufacturing has some shortcomings and problems.
into consideration before further exploring the prospects Choice of materials, production time, low mechanical
Volume 10 Issue 2 (2024) 96 doi: 10.36922/ijb.1637