Page 378 - IJB-10-2
P. 378
International Journal of Bioprinting AM evaluation of medical device companies
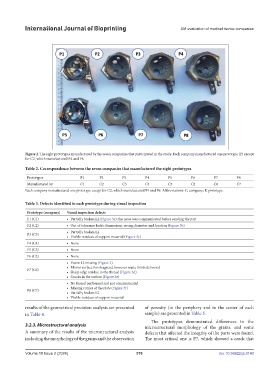
Figure 2. The eight prototypes manufactured by the seven companies that participated in the study. Each company manufactured one prototype (P) except
for C2, which manufactured P4 and P6.
Table 2. Correspondence between the seven companies that manufactured the eight prototypes
Prototypes P1 P2 P3 P4 P5 P6 P7 P8
Manufactured by C1 C2 C3 C4 C5 C2 C6 C7
Each company manufactured one prototype except for C2, which manufactured P4 and P6. Abbreviations: C, company; P, prototype.
Table 3. Defects identified in each prototype during visual inspection
Prototype (company) Visual inspection defects
P1 (C1) • Partially broken L2 (Figure 3a); the issue was communicated before sending the part
P2 (C2) • Out of tolerance hole’s dimensions, wrong diameter and location (Figure 3b)
• Partially broken L2
P3 (C3)
• Visible residues of support material (Figure 3c)
P4 (C4) • None
P5 (C5) • None
P6 (C2) • None
• Entire L2 missing (Figure 2)
• Mirror surface finish agreed, however matte finish delivered
P7 (C6)
• Sharp edge residue in the thread (Figure 3d)
• Cracks in the surface (Figure 3e)
• No thread performed and not communicated
• Missing corner of the plate (Figure 3f)
P8 (C7)
• Partially broken L2
• Visible residues of support material
results of the geometrical precision analysis are presented of porosity (in the periphery and in the center of each
in Table 4. sample) are presented in Table 5.
The prototypes demonstrated differences in the
3.2.3. Microstructural analysis microstructural morphology of the grains, and some
A summary of the results of the microstructural analysis defects that affected the integrity of the parts were found.
including the morphology of the grains and the observation The most critical one is P7, which showed a crack that
Volume 10 Issue 2 (2024) 370 doi: 10.36922/ijb.0140