Page 376 - IJB-10-2
P. 376
International Journal of Bioprinting AM evaluation of medical device companies
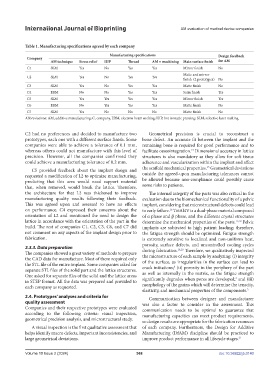
Table 1. Manufacturing specifications agreed by each company
Manufacturing specifications Design feedback
Company
AM technique Stress relief HIP Thread AM + machining Main surface finish for AM
C1 SLM Yes No Yes Yes Mirror finish No
Matte and mirror
C2 SLM Yes No Yes Yes
finish (2 prototypes) No
C3 SLM Yes No Yes Yes Matte finish No
C4 EBM No No Yes Yes Satin finish Yes
C5 SLM Yes Yes Yes Yes Mirror finish Yes
C6 EBM No Yes Yes Yes Matte finish No
C7 SLM Yes No No No Matte finish No
Abbreviations: AM, additive manufacturing; C, company, EBM, electron beam melting; HIP, hot isostatic pressing; SLM, selective laser melting.
C2 had no preferences and decided to manufacture two Geometrical precision is crucial to reconstruct a
prototypes, each one with a different surface finish. Some bone defect. An accurate fit between the implant and the
companies were able to achieve a tolerance of 0.1 mm, remaining bone is required for good performance and to
whereas others could not manufacture with this level of facilitate osseointegration. Dimensional accuracy in lattice
13
precision. However, all the companies confirmed they structures is also mandatory as they allow for soft tissue
could achieve a manufacturing tolerance of 0.2 mm. adherence and vascularization within the implant and affect
15
C5 provided feedback about the implant design and the scaffold mechanical properties. Geometrical deviations
requested a modification of L2 to optimize manufacturing, outside the agreed-upon manufacturing tolerances cannot
predicting that this area would need support material be allowed because non-compliance could possibly cause
that, when removed, would break the lattice. Therefore, some risks to patients.
the architecture for that L2 was thickened to improve The internal integrity of the parts was also critical in the
manufacturing quality results following their feedback. evaluation due to the biomechanical functionality of a pelvic
This was agreed upon and assessed to have no effects implant, considering that microstructural defects could lead
on performance. C4 expressed their concerns about the to early failure. Ti6Al4V is a dual-phase material composed
16
orientation of L2 and mentioned the need to design the of α phase and β phase, and the different crystal structures
lattice in accordance with the orientation of the part in the determine the mechanical properties of the parts. 17,18 Pelvic
build. The rest of companies C1, C2, C3, C6, and C7 did implants are subjected to high patient loading; therefore,
not comment on any aspects of the implant design prior to the fatigue strength should be optimized. Fatigue strength
fabrication. is extremely sensitive to localized and non-uniform heat,
porosity, surface defects, and uncontrolled cooling cycles
2.3.3. Data preparation 19,20
The companies showed a great variety of methods to prepare during fabrication. Therefore, we qualitatively inspected
the CAD data for manufacture. Most of them required only the microstructure of each sample by analyzing: (i) integrity
the STL file of the entire implant. Some companies asked for of the surface, as irregularities in the surface can lead to
8
separate STL files of the solid part and the lattice structures. crack initiations; (ii) porosity in the periphery of the part
One asked for separate files of the solid and the lattice areas as well as internally in the matrix, as the fatigue strength
3
in STEP format. All the data was prepared and provided to significantly degrades when pores are developed; and (iii)
each company as requested. morphology of the grains which will determine the tenacity,
elasticity, and mechanical properties of the components. 3
2.4. Prototypes’ analyses and criteria for Communication between designer and manufacturer
quality assessment was also a factor to consider in the assessment. This
Companies and their respective prototypes were evaluated communication needs to be optimal to guarantee that
according to the following criteria: visual inspection, manufacturing capacities can meet product requirements,
geometrical precision analysis, and microstructural study.
so design results are appropriate for the fabrication resources
A visual inspection is the first qualitative assessment that of each company. Furthermore, the Design for Additive
helps identify macro defects, important inconsistencies, and Manufacturing (DfAM) discipline should be practiced to
large geometrical deviations. improve product performance in all lifecycle stages.
21
Volume 10 Issue 2 (2024) 368 doi: 10.36922/ijb.0140