Page 245 - IJB-10-3
P. 245
International Journal of Bioprinting Increased ECM stiffness enhances chemoresistance
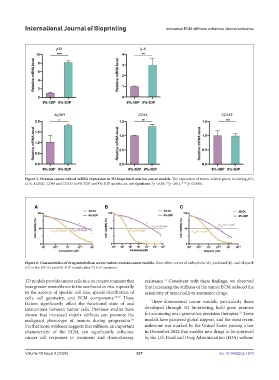
Figure 5. Ovarian cancer-related mRNA expression in 3D-bioprinted ovarian cancer models. The expression of tumor-related genes, including p53,
IL-6, ALDH1, CD44 and CD133 in 6%-3DP and 8%-3DP models. ns, not significant; *p <0.05; **p <0.01; ****p <0.0001.
Figure 6. Characteristics of drug metabolism across various ovarian cancer models. Dose-effect curves of carboplatin (A), paclitaxel (B), and Olaparib
(C) in the 2D-Ov and 6%-3DP models after 72 h of treatment.
3D models provide tumor cells in a microenvironment that resistance. Consistent with these findings, we observed
51
bear greater resemblance to the one found in vivo, especially that increasing the stiffness of the tumor ECM reduced the
in the aspects of specific cell size, spatial distribution of sensitivity of tumor cells to antitumor drugs.
cells, cell geometry, and ECM components. 48,49 These
factors significantly affect the functional state of and Three-dimensional tumor models, particularly those
interactions between tumor cells. Previous studies have developed through 3D bioprinting, hold great promise
52
shown that increased matrix stiffness can promote the for advancing next-generation precision therapies. These
malignant phenotype of tumors during progression. models have garnered global support, and the most recent
50
Furthermore, evidence suggests that stiffness, an important milestone was marked by the United States passing a law
characteristic of the ECM, can significantly influence in December 2022 that enables new drugs to be approved
cancer cell responses to treatment and chemotherapy by the U.S. Food and Drug Administration (FDA) without
Volume 10 Issue 3 (2024) 237 doi: 10.36922/ijb.1673