Page 560 - IJB-10-3
P. 560
International Journal of Bioprinting Engineered 3D-printed PVA vascular grafts
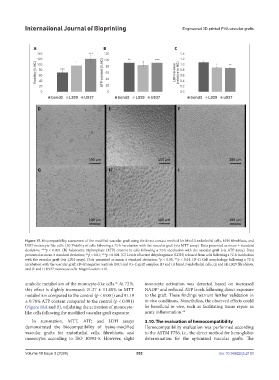
Figure 15. Biocompatibility assessment of the modified vascular graft using the direct contact method for bEnd.3 endothelial cells, L929 fibroblasts, and
U937 monocyte-like cells. (A) Viability of cells following a 72-h incubation with the vascular graft (via MTT assay). Data presented as mean ± standard
deviation; ***p < 0.001. (B) Adenosine triphosphate (ATP) content in cells following a 72-h incubation with the vascular graft (via ATP assay). Data
presented as mean ± standard deviation; **p < 0.01; ***p < 0.001. (C) Levels of lactate dehydrogenase (LDH) released from cells following a 72-h incubation
with the vascular graft (via LDH assay). Data presented as mean ± standard deviation; *p < 0.05; **p < 0.01. (D–I) Cell morphology following a 72-h
incubation with the vascular graft: (D–F) negative controls (NC) and (G–I) graft samples; (D and G) bEnd.3 endothelial cells, (E and H) L929 fibroblasts,
and (F and I) U937 monocyte cells. Magnification: ×10.
66
anabolic metabolism of the monocyte-like cells. At 72 h, monocyte activation was detected based on increased
+
this effect is slightly increased: 21.27 ± 11.48% in MTT NADP and reduced ATP levels following direct exposure
metabolism compared to the control (p < 0.001) and 91.19 to the graft. These findings warrant further validation in
± 0.78% ATP content compared to the control (p < 0.001) in vivo conditions. Nonetheless, the observed effects could
(Figure 16A and B), validating the activation of monocyte- be beneficial in vivo, such as facilitating tissue repair in
like cells following the modified vascular graft exposure. acute inflammation. 68
In summation, MTT, ATP, and LDH assays 3.10. The evaluation of hemocompatibility
demonstrated the biocompatibility of lysine-modified Hemocompatibility evaluation was performed according
vascular grafts for endothelial cells, fibroblasts, and to the ASTM F756, i.e., the direct method for hemoglobin
monocytes according to ISO 10993-5. However, slight determination for the optimized vascular grafts. The
Volume 10 Issue 3 (2024) 552 doi: 10.36922/ijb.2193