Page 557 - IJB-10-3
P. 557
International Journal of Bioprinting Engineered 3D-printed PVA vascular grafts
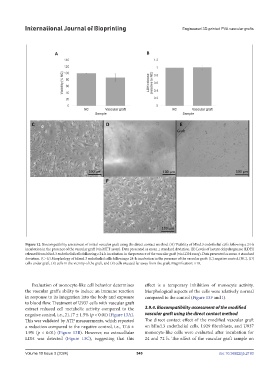
Figure 12. Biocompatibility assessment of initial vascular graft using the direct contact method. (A) Viability of bEnd.3 endothelial cells following a 24-h
incubation in the presence of the vascular graft (via MTT assay). Data presented as mean ± standard deviation. (B) Levels of lactate dehydrogenase (LDH)
released from bEnd.3 endothelial cells following a 24-h incubation in the presence of the vascular graft (via LDH assay). Data presented as mean ± standard
deviation. (C–G) Morphology of bEend.3 endothelial cells following a 24-h incubation in the presence of the vascular graft: (C) negative control (NC), (D)
cells under graft, (E) cells in the vicinity of the graft, and (F) cells situated far away from the graft. Magnification: ×10.
Evaluation of monocyte-like cell behavior determines effect is a temporary inhibition of monocyte activity.
the vascular graft’s ability to induce an immune reaction Morphological aspects of the cells were relatively normal
in response to its integration into the body and exposure compared to the control (Figure 13F and I).
to blood flow. Treatment of U937 cells with vascular graft
extract reduced cell metabolic activity compared to the 3.9.4. Biocompatibility assessment of the modified
negative control, i.e., 21.17 ± 1.5% (p < 0.01) (Figure 13A). vascular graft using the direct contact method
This was validated by ATP measurements, which reported The direct contact effect of the modified vascular graft
a reduction compared to the negative control, i.e., 17.6 ± on bEnd.3 endothelial cells, L929 fibroblasts, and U937
1.9% (p < 0.01) (Figure 13B). However, no extracellular monocyte-like cells were evaluated after incubation for
LDH was detected (Figure 13C), suggesting that this 24 and 72 h. The effect of the vascular graft sample on
Volume 10 Issue 3 (2024) 549 doi: 10.36922/ijb.2193