Page 191 - IJB-10-4
P. 191
International Journal of Bioprinting Effects of structure on the interbody cage
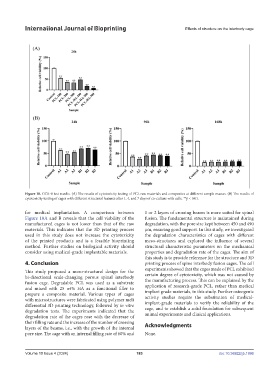
Figure 10. CCK-8 test results. (A) The results of cytotoxicity testing of PCL raw materials and composites at different sample masses. (B) The results of
cytotoxicity testing of cages with different structural features after 1, 4, and 7 days of co-culture with cells. **p < 0.01.
for medical implantation. A comparison between 1 or 2 layers of crossing beams is more suited for spinal
Figure 10A and B reveals that the cell viability of the fusion. The fundamental structure is maintained during
manufactured cages is not lower than that of the raw degradation, with the pore size kept between 450 and 490
materials. This indicates that the 3D printing process μm, ensuring good support. In this study, we investigated
used in this study does not increase the cytotoxicity the degradation characteristics of cages with different
of the printed products and is a feasible bioprinting meso-structures and explored the influence of several
method. Further studies on biological activity should structural characteristic parameters on the mechanical
consider using medical-grade implantable materials. properties and degradation rate of the cages. The aim of
this study is to provide reference for the structure and 3D
4. Conclusion printing process of spine interbody fusion cages. The cell
This study proposed a meso-structural design for the experiments showed that the cages made of PCL exhibited
bi-directional scale-changing porous spinal interbody certain degree of cytotoxicity, which was not caused by
fusion cage. Degradable PCL was used as a substrate the manufacturing process. This can be explained by the
and mixed with 25 wt% HA as a functional filler to application of research-grade PCL, rather than medical
prepare a composite material. Various types of cages implant grade materials, in this study. Further osteogenic
with microstructures were fabricated using polymer melt activity studies require the substitution of medical-
differential 3D printing technology, followed by in vitro implant-grade materials to verify the reliability of the
degradation tests. The experiments indicated that the cage, and to establish a solid foundation for subsequent
degradation rate of the cages rose with the decrease of animal experiments and clinical applications.
their filling rate and the increase of the number of crossing Acknowledgments
layers of the beams, i.e., with the growth of the internal
pore size. The cage with an internal filling rate of 60% and None.
Volume 10 Issue 4 (2024) 183 doi: 10.36922/ijb.1996