Page 364 - IJB-10-4
P. 364
International Journal of Bioprinting 3D-printed PEEK in cranioplasty
Figure 1. Schematic of the manufacturing process of the poly-ether-ether-ketone (PEEK) implant. (a) Schematic of the fused filament fabrication (FFF) of
a PEEK skull implant. (b) The PEEK implant was manufactured by the concentric path-filling method. (c) Schematic diagram of a PEEK prosthetic skull
printed by FFF.
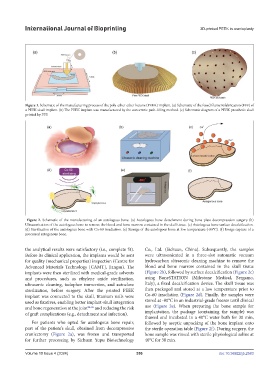
Figure 2. Schematic of the manufacturing of an autologous bone. (a) Autologous bone detachment during bone plate decompression surgery (b)
Ultrasonication of the autologous bone to remove the blood and bone marrow contained in the skull tissue. (c) Autologous bone surface decalcification.
(d) Sterilization of the autologous bone with Co-60 irradiation. (e) Storage of the autologous bone at low temperature (-80°C). (f) Image capture of a
processed autogenous bone.
the analytical results were satisfactory (i.e., complete fit). Co., Ltd. (Sichuan, China). Subsequently, the samples
Before its clinical application, the implants would be sent were ultrasonicated in a three-slot automatic vacuum
for quality (mechanical properties) inspection (Centre for hydrocarbon ultrasonic cleaning machine to remove the
Advanced Materials Technology [CAMT], Jiangsu). The blood and bone marrow contained in the skull tissue
implants were then sterilized with medical-grade solvents (Figure 2b), followed by surface decalcification (Figure 2c)
and procedures, such as ethylene oxide sterilization, using BoneSTATION (Milestone Medical, Bergamo,
ultrasonic cleaning, iodophor immersion, and autoclave Italy), a fixed decalcification device. The skull tissue was
sterilization, before surgery. After the printed PEEK then packaged and stored at a low temperature prior to
implant was connected to the skull, titanium nails were Co-60 irradiation (Figure 2d). Finally, the samples were
used as fixatives, enabling better implant-skull integration stored at -80°C in an industrial-grade freezer until clinical
and bone regeneration at the joint 24-26 and reducing the risk use (Figure 2e). When preparing the bone sample for
of graft complications (e.g., detachment and infection). implantation, the package (containing the sample) was
thawed and incubated in a 40°C water bath for 30 min,
For patients who opted for autologous bone repair, followed by aseptic unpacking of the bone implant onto
part of the patient’s skull, obtained from decompressive the sterile operation table (Figure 2f). During surgery, the
craniectomy (Figure 2a), was frozen and transported bone sample was rinsed with sterile physiological saline at
for further processing by Sichuan Yupu Biotechnology 40°C for 30 min.
Volume 10 Issue 4 (2024) 356 doi: 10.36922/ijb.2583