Page 443 - IJB-10-4
P. 443
International Journal of Bioprinting Improving ductility of 3D-printed Zn–Mg
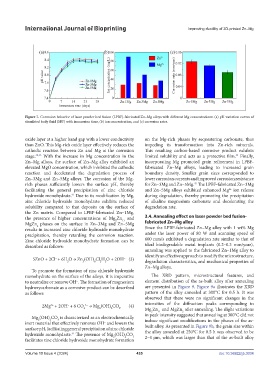
Figure 7. Corrosion behavior of laser powder bed fusion (LPBF)-fabricated Zn–Mg alloys with different Mg concentrations: (a) pH variation curves of
simulated body fluid (SBF) with immersion time, (b) ion concentration, and (c) corrosion rates.
oxide layer at a higher band gap with a lower conductivity on the Mg-rich phases by sequestering carbonate, thus
than ZnO. This Mg-rich oxide layer effectively reduces the impeding its transformation into Zn-rich minerals.
cathodic reaction between Zn and Mg at the corrosion This resulting carbon-based corrosive product exhibits
stage. 42,43 With the increase in Mg concentration in the limited solubility and acts as a protective film. Finally,
44
Zn–Mg alloys, the surface of Zn–Mg alloy exhibited an incorporating Mg promoted grain refinement in LPBF-
elevated MgO concentration, which inhibited the cathodic fabricated Zn–Mg alloys, leading to increased grain
reaction and decelerated the degradation process of boundary density. Smaller grain sizes corresponded to
Zn–3Mg and Zn–5Mg alloys. The corrosion of the Mg- lower corrosion currents and improved corrosion resistance
rich phases sufficiently lowers the surface pH, thereby for Zn–3Mg and Zn–5Mg. The LPBF-fabricated Zn–3Mg
45
facilitating the general precipitation of zinc chloride and Zn–5Mg alloys exhibited enhanced Mg ion release
2+
hydroxide monohydrate. Due to its modification by Mg, during degradation, thereby promoting the precipitation
41
zinc chloride hydroxide monohydrate exhibits reduced of alkaline magnesium carbonate and decelerating the
solubility compared to that deposits on the surface of degradation rate.
the Zn matrix. Compared to LPBF-fabricated Zn–1Mg,
the presence of higher concentrations of Mg Zn and 3.4. Annealing effect on laser powder bed fusion-
11
2
MgZn phases on the surface in Zn–3Mg and Zn–5Mg fabricated Zn–Mg alloy
2
results in increased zinc chloride hydroxide monohydrate Since the LPBF-fabricated Zn–Mg alloy with 1 wt% Mg
precipitation, thereby retarding the corrosion reaction. under the laser power of 80 W and scanning speed of
Zinc chloride hydroxide monohydrate formation can be 600 mm/s exhibited a degradation rate similar to that of
described as follows: ideal biodegradable metal implants (0.2–0.3 mm/year),
annealing was applied to the fabricated Zn–1Mg alloy to
identify an effective approach to modify the microstructure,
5ZnO + 2Cl + 6H O → Zn (OH) Cl H O + 2OH (3)
−
−
2 5 8 2 2 degradation characteristics, and mechanical properties of
Zn–Mg alloys.
To promote the formation of zinc chloride hydroxide
monohydrate on the surface of the alloys, it is imperative The XRD pattern, microstructural features, and
to neutralize or remove OH . The formation of magnesium element distribution of the as-built alloy after annealing
−
hydroxycarbonate as a corrosive product can be described are presented in Figure 9. Figure 9a illustrates the XRD
as follows: pattern of the alloy annealed at 300°C for 0.5 h. It was
observed that there were no significant changes in the
intensities of the diffraction peaks corresponding to
2+
2−
2Mg + 2OH + 6 CO → Mg (OH) CO . (4)
−
2 2 2 3 Mg Zn and MgZn after annealing. The slight variations
2
2
11
in peak intensity suggested that annealing at 300°C did not
Mg (OH) CO is characterized as an electrochemically
3
2
2
inert material that effectively removes OH and lowers the induce significant modifications in the phases of the as-
−
built alloy. As presented in Figure 9b, the grain size within
surface pH, facilitating general precipitation of zinc chloride the alloy annealed at 250°C for 0.5 h was observed to be
hydroxide monohydrate. The presence of Mg (OH) CO 2–4 μm, which was larger than that of the as-built alloy
41
3
2
2
facilitates zinc chloride hydroxide monohydrate formation
Volume 10 Issue 4 (2024) 435 doi: 10.36922/ijb.3034