Page 563 - IJB-10-4
P. 563
International Journal of Bioprinting Light-based muscle bioprinting with bioglass
Fisher, USA) according to manufacturers’ instructions. In modifications to the hardware of the original printer. For
brief, the constructs were washed with 1× PBS and then instance, the original 3D printer employed 300 mL of
fixed with 4% paraformaldehyde (Sigma-Aldrich, USA) resin to operate, which is typically a large printing volume
for 40 min. Later, constructs were soaked with actin/ for bioprinting applications. Therefore, the build plate
DAPI working solution at 37°C for 1 h. We removed the and vat were reduced to minimize the reagents required
actin/DAPI solution and washed samples with 1× PBS. for bioprinting. The retrofitted build plate consisted of a
Different sections of the constructs were evaluated at stainless steel cylinder with a radius of 25 mm attached to
different experimental times employing Alexa Fluor 647 a stainless steel block by a 4 mm stainless steel screw. The
(excitation/emission 647/665 nm) and DAPI (excitation/ build plate featured four leveling screws. An aluminum
emission 360/460 nm) filters. We performed Z-stack and frame, a fluorinated ethylene propylene (FEP) film, and a
orthogonal configuration of the constructs consisting of 3D-printed enclosure were assembled for the customized
30–40 layers with 10 µm in thickness each. Cell alignment vat . The customized apparatus had a 1963 mm bioprinting
2
and morphology were estimated using ImageJ and area and a 17.7 mL sterilizable vat capacity for bioinks. The
Matlab packages. newly designed elements were fabricated in aluminum and
2.9. Statistical analysis High-Temp Resin V02 (Formlabs, Somerville, MA, USA)
A two-way analysis of variance (ANOVA) was conducted using a commercial bioprinter (Formlabs, Somerville, MA,
to analyze variance. To assess statistical differences between USA). These characteristics facilitated the operation of
two independent groups across multiple comparisons, sterilization and handling of the printing materials. The
an unpaired t-test was employed. GraphPad Prism 8 was volume consumption per process was further reduced to
employed to perform data analysis. In this study, mean 2.5 mL for the bioprinting assessments (Figure 1).
differences indicated by p-values of less than 0.05 and 3.2. Tensile strength test
0.001 were considered significant. The mechanical properties of the cell matrix, the biomaterial
where the cells are expected to grow and mature into tissue,
3. Results and discussion are critical in muscle tissue engineering. 21,31 Therefore, we
3.1. Retrofitting for volume consumption reduction first conducted a basic characterization of the mechanical
In a previous report, we described the development properties of materials to be used in our printing
of an LBB system using an affordable commercial 3D experiments. The geometry and dimensions of the samples
laser printer. In this study, we implemented significant used for conducting the tensile tests are presented in Figure
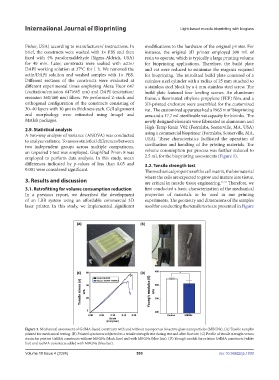
Figure 3. Mechanical assessment of GelMA-based constructs with and without mesoporous bioactive glass nanoparticles (MBGNs). (A) Tensile samples
printed for mechanical testing. (B) Printed specimen subjected to a tensile strength test during test and after fracture. (C) Profile of tensile strength versus
strain for pristine GelMA constructs without MBGNs (black line) and with MBGNs (blue line). (D) Young’s moduli for pristine GelMA constructs (white
bar) and GelMA constructs added with MBGNs (blue bar).
Volume 10 Issue 4 (2024) 555 doi: 10.36922/ijb.1830