Page 118 - IJB-5-1
P. 118
Khan Z, et al.
loaded into a microfluidics tube which fed the liquid into
the coaxial nozzle. Similarly, another 1 mL of serum-
free medium was loaded into a syringe which helped to
dispense the preloaded cells from the nozzle.
After completing the bioprinting process, the printed cylinder
was submerged with complete medium and then incubated
at 37°C, 95% humidity, and 5% CO . Consequently, the a b
2
cylinder was cultured for 2 weeks, to enable the cells to
grow and to stretch in all directions (Figure 2a-d).
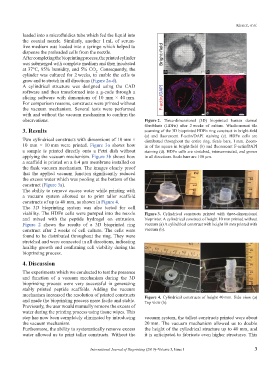
A cylindrical structure was designed using the CAD
software and then transformed into a g-code through a
slicing software with dimensions of 10 mm × 40 mm.
For comparison reasons, constructs were printed without
the vacuum mechanism. Several tests were performed
with and without the vacuum mechanism to confirm the c d
observations. Figure 2. Three-dimensional (3D) bioprinted human dermal
fibroblasts (HDFn) after 2 weeks of culture. Whole-mount tile
3. Results scanning of the 3D bioprinted HDFn ring construct in bright-field
(a) and fluorescent F-actin/DAPI staining (c). HDFn cells are
Two cylindrical constructs with dimensions of 10 mm × distributed throughout the entire ring. Scale bars, 1 mm. Zoom-
10 mm × 10 mm were printed. Figure 3a shows how in of the square in bright-field (b) and fluorescent F-actin/DAPI
a sample is printed directly onto a Petri dish without staining (d). HDFn cells are stretched, interconnected, and grown
applying the vacuum mechanism. Figure 3b shows how in all directions. Scale bars are 100 µm.
a scaffold is printed on a 0.4 µm membrane installed on
the flask vacuum mechanism. The images clearly proof
that the applied vacuum function significantly reduced
the excess water which was pooling at the bottom of the
construct (Figure 3a).
The ability to remove excess water while printing with
a vacuum system allowed us to print taller scaffold
constructs of up to 40 mm, as shown in Figure 4.
The 3D bioprinting system was also tested for cell a b
viability. The HDFn cells were pumped into the nozzle Figure 3. Cylindrical constructs printed with three-dimensional
and mixed with the peptide hydrogel on extrusion. bioprinter. A cylindrical construct of height 10 mm printed without
Figure 2 shows the results of a 3D bioprinted ring vacuum (a) A cylindrical construct with height 10 mm printed with
construct after 2 weeks of cell culture. The cells were vacuum (b).
found to be distributed throughout the ring. They were
stretched and were connected in all directions, indicating
healthy growth and confirming cell viability during the
bioprinting process.
4. Discussion
The experiments which we conducted to test the presence
and function of a vacuum mechanism during the 3D
bioprinting process were very successful in generating
stably printed peptide scaffolds. Adding the vacuum a b
mechanism increased the resolution of printed constructs Figure 4. Cylindrical constructs of height 40 mm. Side view (a)
and made the bioprinting process more facile and stable. Top view (b).
Previously, the user would manually remove the excess of
water during the printing process using tissue wipes. This
step has now been completely eliminated by introducing vacuum system, the tallest constructs printed were about
the vacuum mechanism. 20 mm. The vacuum mechanism allowed us to double
Furthermore, the ability to systematically remove excess the height of the cylindrical structure up to 40 mm, and
water allowed us to print taller constructs. Without the it is anticipated to fabricate even higher structures. This
International Journal of Bioprinting (2019)–Volume 5, Issue 1 3