Page 112 - IJB-10-5
P. 112
International Journal of Bioprinting 3D bioprinting for organoid-derived EVs
implants for bone regeneration and joint organoids cancer development and precision oncology, facilitating
that mimic natural joint tissues, offering effective the discovery of patient-specific drugs. 90
treatment options. 52,85,86 Moreover, 3D bioprinting has Despite the challenges associated with conventional
been instrumental in creating tumor models and brain PDO models, the application of 3D bioprinting has shown
organoids for personalized drug screening and disease promise in enhancing organoid culture and addressing
10
modeling. For example, embedded 3D bioprinting and existing limitations, ultimately paving the way for more
photo-crosslinkable bioinks were invented to create brain- accurate and sophisticated models in precision medicine
like co-culture constructs with heterogeneous neuronal (Figure 3).
populations, demonstrating their potential for neuronal 2.4. Applications of 3D bioprinting of organoids in
87
differentiation and modeling neurological diseases. studying inflammation
This innovative approach has led to the development of Organoids, particularly those generated through 3D
bioprinted brain organoids for individual drug screening bioprinting, have emerged as valuable tools for studying
in neurological diseases. 88 inflammatory diseases and cancer immunotherapy. By
In cancer research, acoustically bioprinted patient- incorporating immune cells, stromal cells, and other
derived microtissues have been developed to model cancer relevant cell types into organoid models, the complex
invasion and predict treatment responses in colorectal cellular interactions and microenvironments observed
in autoimmune diseases can be recapitulated. These
cancer patients, showcasing the personalized and models enable the exploration of disease mechanisms,
predictive capabilities of 3D bioprinting. Flores-Torres such as the impact of specific genes and signaling
89
et al. established bioprinted tumor models to maintain the pathways in inflammation, and facilitate the screening of
PDO of gastric adenocarcinoma using hydrogels composed potential therapeutic interventions. Table 3 summarizes
of alginate and gelatin. Additionally, bioprinted iPSC- the literature related to 3D bioprinting organoids and
88
derived cancer tissues offer opportunities to study early inflammatory diseases.
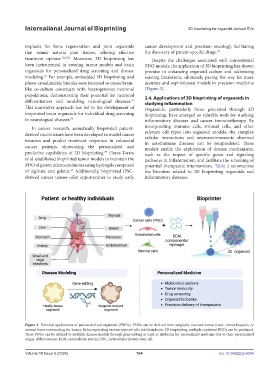
Figure 3. Potential applications of patient-derived organoids (PDOs). PDOs can be derived from surgically resected tumor tissue, tumor biopsies, or
normal tissue surrounding the tumor. By incorporating various types of cells into bioinks for 3D bioprinting, multiple consistent PDOs can be produced.
These PDOs can be utilized to establish disease models through gene editing or used as platforms for personalized medicine due to their personalized
origin. Abbreviations: ECM, extracellular matrix; iPSC, induced pluripotent stem cell.
Volume 10 Issue 5 (2024) 104 doi: 10.36922/ijb.4054