Page 12 - IJB-10-5
P. 12
International Journal of Bioprinting 3D bioprinting for nanoparticle evaluation
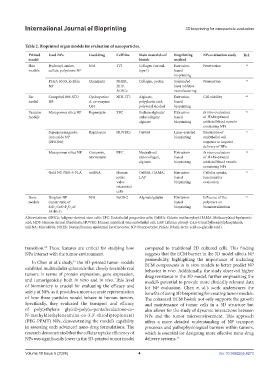
Table 2. Bioprinted organ models for evaluation of nanoparticles.
Printed Used NPs Used drug Cell line Main materials of Bioprinting NPs evaluation study Ref.
model bioink method
Skin Hydroxyl, amine, N/A 3T3 Collagen (rat rail, Extrusion- Penetration 56
models sulfate, polystrene NP type I) based
bioprinting
PLGA 50:50, lecithin Quinizarin NHEK, Collagen, pectin Suspended Permeation 57
NP HDF, layer additive
ADSCs manufacturing
Ear Compritol 888 ATO Cyclosporine NIH-3T3 Alginate, Extrusion- Cell viability 62
model NP A, co-enzyme polyylactic acid, based
Q10 polyvinyl alcohol bioprinting
Vascular Mesoporous silica NP Rapamycin EPC Sodium alginate/ Extrusion- In vivo evaluation 71
models atelocollagen/ based of 3D-bioprinted
alginate bioprinting artificial blood vessels
containing NPs
Superparamagnetic Rapamycin HUVECs GelMA Laser-assisted Evaluation of 75
iron oxide NP bioprinting endothelial cell
(SPIONs) response to targeted
delivery of NPs
Mesoporous silica NP Curcumin, EPC Neutralized Extrusion- In vivo evaluation 76
atorvastatin atelocollagen, based of 3D-bioprinted
alginate bioprinting artificial blood vessels
containing NPs
Gold NP, PEG-b-PLA miRNA Human GelMA, HAMA, Extrusion- Cellular uptake, 79
aortic LAP based functionality
valve bioprinting evaluation
interstitial
cells
Bone Bioglass NP N/A SaOS-2 Alginate/gelatin Extrusion- Influence of the 92
models (molar ratio of based polymers on
SiO ∶CaO∶P O of bioprinting biomineralization
2
2
5
55∶40∶5)
Abbreviations: ADSCs: Adipose-derived stem cells; EPC: Endothelial progenitor cells; GelMA: Gelatin methacryloyl; HAMA: Methacrylated hyaluronic
acid; HDF: Human dermal fibroblasts; HUVEC: Human umbilical vein endothelial cell; LAP: Lithium phenyl-2,4,6-trimethylbenzoylphosphinate;
miRNA: MicroRNA; NHEK: Normal human epidermal keratinocytes; NP: Nanoparticle; PLGA: Poly(L-lactic acid-co-glycolic acid).
transition. These features are critical for studying how compared to traditional 2D cultured cells. This finding
32
NPs interact with the tumor environment. suggests that the ECM barrier in the 3D model affects NP
permeability, highlighting the importance of including
In Chen et al.’s study, the 3D-printed tumor models
33
exhibited multicellular spheroids that closely resemble real ECM components in in vitro models to better predict NP
behavior in vivo. Additionally, the study observed higher
tumors in terms of protein expression, gene expression, drug resistance in the 3D model, further emphasizing the
and tumorigenicity both in vitro and in vivo. This level model’s potential to provide more clinically relevant data
of biomimicry is crucial for evaluating the efficacy and for NP evaluation. Chen et al.’s work underscores the
safety of NPs, as it provides a more accurate representation benefits of using 3D bioprinting for creating tumor models.
of how these particles would behave in human tumors. The enhanced ECM bioink not only supports the growth
Specifically, they evaluated the transport and efficacy and maintenance of tumor cells in a 3D structure but
of poly(ethylene glycol)-poly(ω-pentadecalactone-co- also allows for the study of dynamic interactions between
N-methyldiethyleneamine-co-3,3’-thiodipropionate) NPs and the tumor microenvironment. This approach
(PEG-PPMT) NPs, demonstrating the model’s capability offers a more detailed understanding of NP transport
in assessing such advanced nano-drug formulations. The processes and pathophysiological barriers within tumors,
research demonstrated that the cellular uptake efficiency of which is essential for designing more effective nano-drug
NPs was significantly lower in the 3D-printed tumor model delivery systems. 33
Volume 10 Issue 5 (2024) 4 doi: 10.36922/ijb.4273