Page 26 - IJB-10-5
P. 26
International Journal of Bioprinting 3D bioprinting for nanoparticle evaluation
in the outer matrix of these scaffolds provided additional 7.1. Three-dimensional bioprinted colitis-mimicking
mechanical support and bioactivity, leading to improved model for evaluation of albumin nano-encapsulated
outcomes in terms of cell proliferation and mineralization. anti-inflammatory drugs
By incorporating bioglass into the bioprinted scaffolds, Almutary et al. conducted an insightful study leveraging
101
the study demonstrates a significant enhancement in the 3D bioprinting technology to develop a colitis-mimicking
biomineralization capabilities of SaOS-2 cells, providing model, aiming to assess epithelial barrier function using
a valuable strategy for the development of advanced, albumin nano-encapsulated anti-inflammatory drugs. The
biocompatible implants. 92 research addresses a significant gap in drug development:
the lack of effective preclinical models that accurately
7. Three-dimensional bioprinted inflamma- replicate the physiological conditions of the human
tory disease model intestine, which often leads to poor predictions of drug
efficacy and toxicity. The study utilized 3D bioprinting
Three-dimensional bioprinting technology has emerged to create a model that closely mimics the intestinal
as a crucial tool in the study of inflammatory diseases. 93–95 environment under colitis conditions. The authors
This technology enables the creation of models that employed Caco-2 and HT-29 colon cancer cell lines, which
surpass traditional 2D culture systems by replicating the are standard in studies of intestinal function. These cells
complex structures and environments of actual human were incorporated into a bioink and bioprinted using the
96
tissues. Such models allow for a more precise analysis INKREDIBLE bioprinter, which offers precise control
of inflammatory responses and interactions between over the printing process. The bioprinter used pneumatic
immune cells. They are particularly useful in studying pressure to extrude cell-laden hydrogel strands, creating a
inflammatory bowel diseases, Crohn’s disease, colitis, layered structure that closely resembles the architecture of
and other related conditions, facilitating the evaluation the intestinal epithelium. One of the key advantages of 3D
of new anti-inflammatory treatments. 97–99 By accurately bioprinting highlighted in the study is its ability to control
mimicking the pathological features of inflammatory cell shape and spatial organization, which are crucial for
diseases, 3D bioprinting enhances the preclinical testing accurate physiological modeling. Traditional 2D cell
100
phase, providing reliable data on the efficacy and safety of cultures fail to replicate the complex interactions and 3D
drugs before they enter clinical trials. structure of tissues, leading to less reliable data. In contrast,
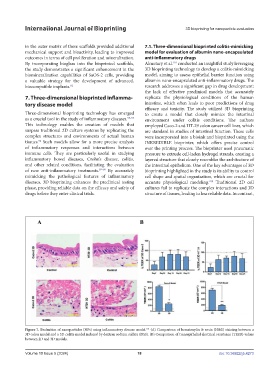
Figure 7. Evaluation of nanoparticles (NPs) using inflammatory disease model. (A) Comparison of hematoxylin & eosin (H&E) staining between a
101
3D colon model and a 3D colitis model induced by dextran sodium sulfate (DSS). (B) Comparison of transepithelial electrical resistance (TEER) values
between 2D and 3D models.
Volume 10 Issue 5 (2024) 18 doi: 10.36922/ijb.4273