Page 463 - IJB-10-5
P. 463
International Journal of Bioprinting 3D-printed plasma devices for decontamination
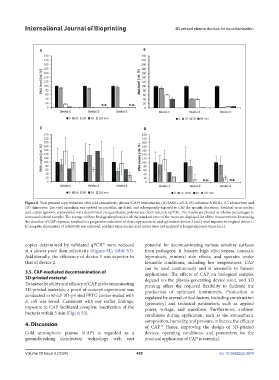
Figure 8. Viral genome copy reduction after cold atmospheric plasma (CAP) treatment for (A) SARS-CoV-2; (B) influenza A H1N1; (C) adenovirus; and
(D) rhinovirus. The viral inoculum was spotted on parafilm, air-dried, and subsequently exposed to CAP for specific durations. Residual virus nucleic
acid copies (genome equivalents) were determined via quantitative polymerase chain reaction (qPCR). The results are plotted as relative percentages to
untreated control samples. The average of three biological replicates with the standard error of the mean are displayed for all the measurements. Increasing
the duration of CAP exposure resulted in a progressive reduction of viral copy numbers, and optimized devices 2 and 3 were superior to original device 1.
If complete elimination of infectivity was achieved, residual virus nucleic acid copies were not analyzed at longer exposure times (n.a.).
copies determined by validated qPCR were reduced potential for decontaminating various sensitive surfaces
56
at a slower pace than infectivity (Figure 8C; Table S3). from pathogens. It features high effectiveness, nontoxic
Additionally, the efficiency of device 3 was superior to byproducts, minimal side effects, and operates under
that of device 2. favorable conditions, including low temperatures. CAP
can be used continuously and is amenable to human
3.5. CAP-mediated decontamination of applications. The effects of CAP on biological samples
3D-printed material depend on the plasma-generating device used, and 3D
To assess the ability and efficacy of CAP in decontaminating printing offers the required flexibility to facilitate the
3D-printed materials, a proof-of-concept experiment was production of optimized instruments. Production is
conducted in which 3D-printed PETG contaminated with regulated by several critical factors, including construction
E. coli was tested. Consistent with our earlier findings, (geometry) and technical parameters, such as applied
exposure to CAP facilitated complete inactivation of the power, voltage, and waveform. Furthermore, ambient
bacteria within 5 min (Figure S3). conditions during application, such as the atmospheric
4. Discussion composition, humidity, and pressure, influence the efficacy
of CAP. Hence, improving the design of 3D-printed
29
Cold atmospheric plasma (CAP) is regarded as a devices, operating conditions, and parameters for the
groundbreaking disinfection technology with vast practical application of CAP is essential.
Volume 10 Issue 5 (2024) 455 doi: 10.36922/ijb.3679