Page 67 - IJB-10-5
P. 67
International Journal of Bioprinting Medical regenerative in situ bioprinting
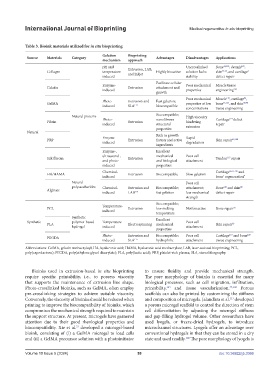
Table 3. Bioink materials utilized for in situ bioprinting
Gelation Bioprinting
Source Materials Category Advantages Disadvantages Applications
mechanism approach
pH and Uncrosslinked Bone 41,105 , dermis ,
109
Collagen temperature- Extrusion, LAB, Highly bioactive solution lacks skin 37,34 , and cartilage 4
and inkjet
induced stability defect repair
Facilitate cellular
Enzyme- Poor mechanical Muscle tissue
Gelatin Extrusion attachment and
induced properties engineering 110
growth
112
56
Poor mechanical Muscle , cartilage ,
Photo- Extrusion and Fast gelation;
GelMA properties at low bone 35,113 , and skin 69,90
induced SLA 111 biocompatible
concentrations tissue engineering
Natural proteins Biocompatible; High viscosity
114
Fibrin Photo- Extrusion nanofibrous hindering Cartilage defect
induced structural extrusion repair
properties
Natural
Enzyme- Rich in growth Rapid
PRP Extrusion factors and active Skin repair 63,108
induced degradation
ingredients
Enzyme-, Excellent
ultrasound-, mechanical Poor cell
Silk fibroin Extrusion Tendon repair
115
and photo- and biological attachment
induced properties
Chemical- Cartilage 66,55,116 and
HA/HAMA Extrusion Biocompatible Slow gelation
1
induced bone regeneration”
Natural Poor cell
polysaccharides Chemical- Extrusion and Biocompatible; attachment; Bone and skin
118
88
Alginate
induced LAB 117 fast gelation low mechanical defect repair
strength
Biocompatible;
Temperature-
PCL Extrusion low melting Not bioactive Bone repair 58
induced
temperature
Synthetic
Synthetic polymer-based Temperature- Excellent Poor cell
PLA Electrospinning mechanical Skin repair 46
hydrogel induced attachment
properties
Photo- Extrusion and Biocompatible; Poor cell Cartilage and bone
119
120
PEGDA
induced SLA 111 hydrophilic attachment tissue engineering
Abbreviations: GelMA, gelatin methacryloyl; HA, hyaluronic acid; HAMA, hyaluronic acid methacrylate; LAB, laser-assisted bioprinting; PCL,
poly(caprolactone); PEGDA, poly(ethylene glycol diacrylate); PLA, poly(lactic acid); PRP, platelet-rich plasma; SLA, stereolithography.
Bioinks used in extrusion-based in situ bioprinting to ensure fluidity and provide mechanical strength.
require specific printability, i.e., to possess viscosity The pore morphology of bioinks is essential for many
that supports the maintenance of extrusion line shape. biological processes, such as cell migration, infiltration,
Photo-crosslinked bioinks, such as GelMA, often employ printability, and tissue vascularization. 77,102 Porous
123
pre-crosslinking strategies to achieve suitable viscosity. scaffolds can also be printed by customizing the stiffness
Conversely, the viscosity of bioinks should be reduced when and composition of microgels. Jalandhra et al. developed
113
printing to improve the biocompatibility of bioinks, which a porous microgel scaffold to control the direction of stem
compromises the mechanical strength required to maintain cell differentiation by adjusting the microgel stiffness
the support structure. At present, microgels have garnered and gap-filling hydrogel volume. Other researchers have
attention due to their good rheological properties and used lyogels, or freeze-dried hydrogels, to introduce
biocompatibility. Xie et al. developed a microgel-based microchannel structures. Lyogels offer an advantage over
73
bioink, consisting of (i) a GelMA microgel to load cells conventional hydrogels in that they can be stored in a dry
and (ii) a GelMA precursor solution with a photoinitiator state and used readily. The pore morphology of lyogels is
102
Volume 10 Issue 5 (2024) 59 doi: 10.36922/ijb.3366