Page 86 - IJB-10-5
P. 86
International Journal of Bioprinting dECM bioink for 3D musculoskeletal tissue reg.
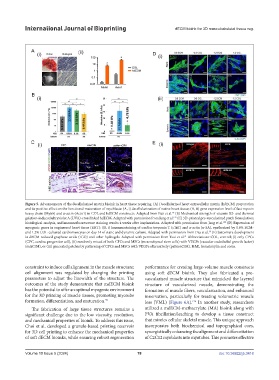
Figure 5. Advancements of the decellularized matrix bioink in heart tissue repairing. (A) Decellularized heart extracellular matrix (hdECM) preparation
and its positive effect on the functional maturation of myoblasts: (A, i) decellularization of native heart tissue; (A, ii) gene expression level of fast myosin
heavy chain (Myh6) and α-actin (Actn1) in COL and hdECM constructs. Adapted from Pati et al. (B) Mechanical strength of vitamin B2- and thermal
24
gelation-induced ultraviolet A (UVA)-crosslinked hdECM. Adapted with permission from Jang et al. (C) 3D-printed pre-vascularized patch formulation;
147
histological analysis, and immunofluorescence staining results 4 weeks after implantation. Adapted with permission from Jang et al. (D) Expression of
120
myogenic genes in engineered heart tissue (EHT): (D, i) Immunostaining of cardiac troponin T (cTnT) and α-actin (α-SA), synthesized by 0.6% ECM-
and 1.2% COL-cultured cardiomyocytes on day 14 of static and dynamic culture. Adapted with permission from Das et al. (E) Sarcomere development
38
in dECM-reduced graphene oxide (rGO) and other hydrogels. Adapted with permission from Tsui et al. Abbreviations: COL, control; (i) only CPCs
41
(CPC, cardiac progenitor cell), (ii) randomly mixed of both CPCs and MSCs (mesenchymal stem cells) with VEGFs (vascular endothelial growth factor)
(mixC/M), or (iii) generated patches by patterning of CPCs and MSCs with VEGFs alternatively (patternC/M). H&E, hematoxylin and eosin.
constraint to induce cell alignment in the muscle structure; performance for creating large-volume muscle constructs
cell alignment was regulated by changing the printing using soft dECM bioink. They also fabricated a pre-
parameters to adjust the linewidth of the structure. The vascularized muscle structure that mimicked the layered
outcomes of the study demonstrate that mdECM bioink structure of vascularized muscle, demonstrating the
has the potential to offer an optimal myogenic environment formation of muscle fibers, vascularization, and enhanced
for the 3D printing of muscle tissues, promoting myotube innervation, particularly for treating volumetric muscle
formation, differentiation, and maturation. loss (VML) (Figure 6A). In another study, researchers
39
124
The fabrication of large tissue structures remains a utilized a mdECM-methacrylate (MA) bioink along with
significant challenge due to the low viscosity, resolution, PVA fibrillation/leaching to develop a tissue construct
and mechanical properties of bioink. To address this issue, that mimics cellular skeletal muscle. This unique approach
Choi et al. developed a granule-based printing reservoir incorporates both biochemical and topographical cues,
for 3D cell printing to enhance the mechanical properties synergistically enhancing the alignment and differentiation
of soft dECM bioinks, while ensuring robust regeneration of C2C12 myoblasts into myotubes. This promotes effective
Volume 10 Issue 5 (2024) 78 doi: 10.36922/ijb.3418