Page 90 - IJB-10-5
P. 90
International Journal of Bioprinting dECM bioink for 3D musculoskeletal tissue reg.
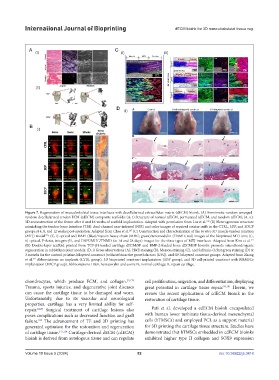
Figure 7. Regeneration of musculoskeletal tissue interfaces with decellularized extracellular matrix (dECM) bioink. (A) Biomimetic random-arranged-
random decellularized tendon ECM (tdECM) composite scaffolds: (A, i) Structure of normal tdECM, permutated tdECM, and random tdECM; (A, ii):
3D-reconstruction of the femur after 8 and 16 weeks of scaffold implantation. Adapted with permission from Liu et al. (B) Heterogeneous structure
168
mimicking the tendon-bone interface (TBI): dual-channel near-infrared (NIR) and color images of repaired rotator cuffs in the CTRL, 3DP, and 3DCP
groups at 4, 8, and 12 weeks post-operation. Adapted from Chae et al. (C) Construction and characterization of the in vitro 3D muscle-tendon junction
167
(MTJ) model : (C, i) optical and DAPI (blue)/myosin heavy chain (MHC; green)/tenomodulin (TNMD; red) images of the bioprinted MTJ unit; (C,
173
ii) optical, F-Actin, integrin-β1, and DAPI/MHC/TNMD (at 14 and 28 days) images for the three types of MTJ interfaces. Adapted from Kim et al.
171
(D) Double-layer scaffold printed from TGF-β1-loaded cartilage dECM/SF and BMP-2-loaded bone dECM/SF bioinks promote osteochondrogenic
regeneration in rabbit knee joint models: (D, i) Gross observations (A), H&E staining (B), Masson staining (C), and Safranin-O/fast green staining (D) at
3 months for the control, pristine-bilayered construct (without bioactive growth factors [GFs]), and GF-bilayered construct groups. Adapted from Zhang
et al. Abbreviations: no implants (CTRL group), 3D bioprinted construct implantation (3DP group), and 3D cell-printed construct with BMMSCs
131
implantation (3DCP group). Abbreviations: H&E, hematoxylin and eosin; N, normal cartilage; R, repair cartilage.
chondrocytes, which produce ECM, and collagen. 29,179 cell proliferation, migration, and differentiation, displaying
Trauma, sports injuries, and degenerative joint diseases great potential in cartilage tissue repair. 99,181 Herein, we
can cause the cartilage tissue to be damaged and worn. review the recent applications of cdECM bioink in the
Unfortunately, due to its vascular and neurological restoration of cartilage tissue.
properties, cartilage has a very limited ability for self-
repair. 24,29 Surgical treatment of cartilage lesions also Pati et al. developed a cdECM bioink encapsulated
poses complications such as decreased function and graft with human lower turbinate tissue-derived mesenchymal
failure. The advancement of TE and 3D printing has cells (hTMSCs) and employed PCL as a support material
180
generated optimism for the restoration and regeneration for 3D printing the cartilage tissue structure. Studies have
of cartilage tissue. 133,134 Cartilage-derived dECM (cdECM) demonstrated that hTMSCs embedded in cdECM bioinks
bioink is derived from autologous tissue and can regulate exhibited higher type II collagen and SOX9 expression
Volume 10 Issue 5 (2024) 82 doi: 10.36922/ijb.3418