Page 87 - IJB-10-5
P. 87
International Journal of Bioprinting dECM bioink for 3D musculoskeletal tissue reg.
recovery of diseased skeletal muscle tissue with highly secretion functions. 152,153 The collagen ECM of tendon
consistent myogenic characteristics (Figure 6B). 125 tissue is assembled in a layered manner, contributing to its
high tensile properties with an ultimate tensile strength of
Additionally, several studies have focused on improving 29,152
3D printing and scaffold manufacturing technology to 50–150 MPa. Minor tendon fiber ruptures can often
heal naturally, but larger injuries require graft implantation
meet clinical needs. 30,151 Researchers have developed a for repair or regeneration. Moreover, the regeneration
technology for the specific treatment of VML by combining capability of autologous or donor graft implantation
freeform reversible embedding of suspended hydrogels repair may be limited with a high risk of re-fracture. 154,155
(FRESH) 3D bioprinting with computed tomography (CT), Despite advancements in regenerative techniques, such as
utilizing dECM and collagen-type I bioink to construct cell therapy, biomaterials, and fine scaffold design, there
large-volume dECM patches with precise control of fiber remains a challenge in achieving artificial tendons with
arrangement (Figure 6C). 151 mechanical properties comparable to natural tissues. 156–160
6.3. Tendon Herein, we review the novel application of tendon dECM
The tendon tissue is composed of various cell types, bioinks in the 3D printing of tendon tissue for tendon
including a dense collagen ECM that connects skeletal regeneration.
muscle to bone and transmits tension during movement. Toprakhisar et al. employed optimized tissue
The most dominant cells in the tendon tissue are tenocytes, decellularization and solubilization methods to extract a
i.e., specialized fibroblasts that regulate tendon ECM biocompatible dECM bioink with rapid gel kinetics from
remodeling through their mechanosensory and collagen a bovine Achilles tendon. Studies have demonstrated
161
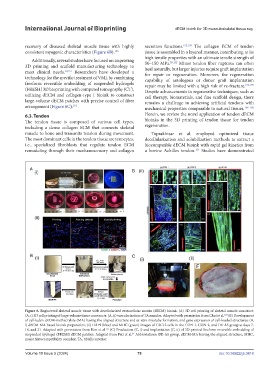
Figure 6. Engineered skeletal muscle tissue with decellularized extracellular matrix (dECM) bioink. (A) 3D cell printing of skeletal muscle construct:
(A, i) 3D cell printing of large-volume tissue constructs; (A, ii) vascularization of TA muscles. Adapted with permission from Choi et al. (B) Development
124
of cell-laden dECM-methacrylate (MA) having the aligned structure and in vitro myotube formation, and gene expression of cell-loaded structures: (B,
i) dECM-MA-based bioink preparation; (ii) DAPI (blue) and MHC (green) images of C2C12 cells in the CON-1, CON-2, and DE-AS group at days 7,
14, and 21. Adapted with permission from Kim et al. (C) Production (C, i) and implantation (C, ii) of 3D-printed freeform reversible embedding of
125
suspended hydrogel (FRESH) dECM patches. Adapted from Pati et al. Abbreviations: DE-AS group, dECM-MA having the aligned structure; MHC,
24
major histocompatibility complex; TA, tibialis anterior.
Volume 10 Issue 5 (2024) 79 doi: 10.36922/ijb.3418