Page 92 - IJB-10-5
P. 92
International Journal of Bioprinting dECM bioink for 3D musculoskeletal tissue reg.
have a porous structure, suitable mechanical properties, with PEGDA/ECM scaffolds. Animal studies demonstrated
and degradation properties. Additionally, these scaffolds that PEGDA/ECM/Hon scaffolds significantly enhanced
can promote MSC proliferation, significantly increase cartilage regeneration. Due to concerns over potential
133
the expression of osteogenesis-related genes (Figure 9C), pathogens in animal-derived dECM, researchers have
and support cartilage regeneration. However, the local developed a new composite bioink named dSCECMMA.
25
immune response presents a challenge that hinders tissue This bioink utilizes decellularized sturgeon cartilage
regeneration. To address this issue, Zhu et al. developed a ECM (dSC-ECM), which is highly similar to human
PEGDA/ECM scaffold containing the natural compound collagen. Modified with methacrylate and combined with
honokiol (Hon). After lipopolysaccharide (LPS) treatment, sericin methacrylate (SerMA), dSCECMMA exhibits
the PEGDA/ECM/Hon scaffold significantly inhibited the good printability and forms a porous structure after light
release of pro-inflammatory cytokines (TNF-α, IL-1β, and curing. In vivo experiments have reported that it effectively
IL-6) from macrophages compared to the control group promoted the regeneration of cartilage tissue. 185
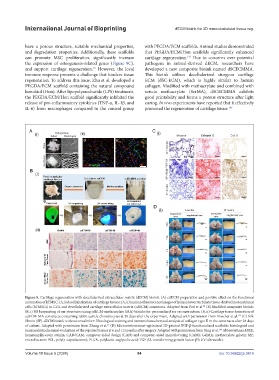
Figure 9. Cartilage regeneration with decellularized extracellular matrix (dECM) bioink. (A) cdECM preparation and positive effect on the functional
maturation of hTMSC: (A, i) decellularization of cartilage tissues; (A, ii) immunofluorescence images of human lower turbinate tissue-derived mesenchymal
24
cells (hTMSCs) in COL and decellularized cartilage extracellular matrix (cdECM) constructs. Adapted from Pati et al. (B) Modified composite bioink:
(B, i) 3D bioprinting of ear structures using cdECM-methacrylate (MA) bioinks for personalized ear reconstruction; (B, ii) Cartilage tissue formation of
136
cdECM-MA constructs containing rabbit auricle chondrocytes at 28 days after the experiment. Adapted with permission from Visscher et al. (C) Silk
fibroin (SF)-dECM bioink without crosslinker: Histological staining and immunohistochemical analysis of collagen type II in the constructs after 28 days
25
of culture. Adapted with permission from Zhang et al. (D) Microenvironment-optimized 3D-printed TGF-β-functionalized scaffolds: histological and
186
immunohistochemical evaluation of the repaired tissues at 6 and 12 months after surgery. Adapted with permission from Yang et al. Abbreviations: H&E,
hematoxylin-eosin stainin; CAD/CAM, computer-aided design (CAD) and computer-aided manufacturing (CAM); GelMA, methacrylate gelatin; MF,
microfracture; PCL, poly(ε-caprolactone); PLGA, polylactic-coglycolic acid; TGF-β3, transforming growth factor-β3; UV: ultraviolet.
Volume 10 Issue 5 (2024) 84 doi: 10.36922/ijb.3418