Page 91 - IJB-10-5
P. 91
International Journal of Bioprinting dECM bioink for 3D musculoskeletal tissue reg.
compared to the control group of hTMSCs embedded in dECM bioinks (PVA-A/SDCM [solubilized decellularized
collagen (Figure 9A). Although the mechanical strength and cartilage matrix] bioink, cdECM-MA bioinks, 136,183
135
biological behavior of the constructs were not measured, aptamer-GE [GelMA/DCECM] bioink, PVA/dECM
184
the potential of cdECM bioinks for cartilage tissue bioink, GelHACS [hyaluronic acid and chondroitin
137
regeneration has been validated. 24,182 Chae et al. developed a sulfate]-MA bioink ) for cartilage bioprinting (Figure 9B).
32
computer-aided design (CAD)-based 3D-printed meniscus
structure using a blend of polyurethane (PU)-PCL cell- To mitigate the potential toxicity from bioink
laden meniscal dECM (me-dECM) bioink. The printed crosslinking (ion crosslinking, photocrosslinking, enzyme
147
constructs displayed excellent mechanical properties crosslinking) and preserve cell viability, Yang et al.
and good biocompatibility, promoting the formation of developed silk fibroin (SF)-dECM bioinks that do not
fibrocartilage via proliferation and differentiation. To require crosslinkers. Instead, they utilized a method
enhance cell viability, bioprintability, and mechanical involving PEG to avoid crosslinking agents and leaching
properties, researchers have developed various composite processes. The scaffolds prepared from SF-dECM bioink
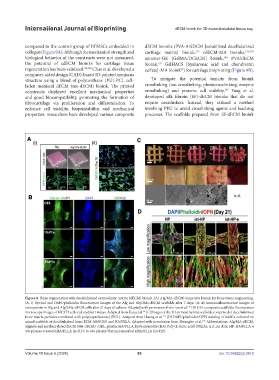
Figure 8. Bone regeneration with decellularized extracellular matrix (dECM) bioink. (A) Alg/MA-dECM composite bioink for bone tissue engineering.
(A, i) Optical and DAPI/phalloidin fluorescence images of the Alg and Alg/2Ma-dECM scaffolds after 7 days; (A, ii) Immunofluorescence images of
osteopontin in Alg and Alg/2Ma-dECM cells after 21 days of culture. Adapted with permission from Lee et al. (B) GEL composite scaffolds: fluorescence
129
microscopy images of MC3T3 cells cultured for 14 days. Adapted from Kara et al. (C) Images of the 3D-printed hybrid scaffolds comprised of decellularized
64
bone matrix particles combined with poly(caprolactone) (PCL). Adapted from Huang et al. (D) DAPI/phalloidin/OPN staining of hASCs cultured on
177
mixed scaffolds of decellularized bone ECM (dbECM) and HA/PLLA. Adapted with permission from Hwangbo et al. Abbreviations: Alg/MA-dECM,
132
alginate and methacrylated dECM (Ma-dECM) ; GEL, gelatin; HA/PLLA, hydroxyapatite (HA) Poly (L-lactic acid) (PLLA); n.d., no data; HP: HA/PLLA n
situ plasma-treated nHA/PLLA (is-HP); In situ plasma/thermal annealed nHA/PLLA (ist-HP).
Volume 10 Issue 5 (2024) 83 doi: 10.36922/ijb.3418