Page 19 - IJB-6-4
P. 19
Shpichka, et al.
(Contd...)
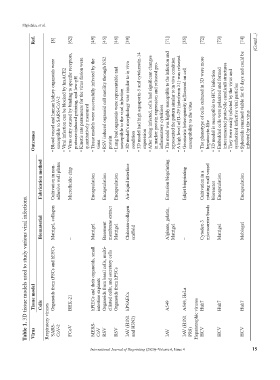
Ref. [8] [62] [49] [45] [46] [39] [71] [55] [72] [73] [74]
susceptible to SARS-CoV-2 Blood vessel and human kidney organoids were • Viral infection can be blocked by hrsACE2 Virus entry caused by binding to specific receptors, protease-induced priming, and low-pH Kinetic rate parameters for the virus fusion were quantitatively measured Tissue models were successfully infected by the RSV induced organoid cell motility through NS2 Lung bud organoids were representable and susceptible to the viral in
Outcomes • • • • virus • protein • • condition • expression • • • • hepatocyte-like • • •
Fabrication method Cultivation in non- adhesive well plates Microfluidic chip Encapsulation Encapsulation Encapsulation Air-liquid interface Extrusion bioprinting Inkjet bioprinting Cultivation in a rotating wall vessel bioreactor Encapsulation Encapsulation
Table 1. 3D tissue models used to study various viral infections.
Biomaterial Matrigel, collagen – Matrigel Basement membrane extract Matrigel Chitosan-collagen scaffold Alginate, gelatin, Matrigel – Cytodex-3 microcarrier beads Matrigel Mebiogel
Organoids from iPSCs and hESCs hPIECs and their organoids, small Organoids from basal cells, multi- ciliated cells, and secretory cells
Tissue model Cells BHK-21 intestine explants Organoids from hPSCs hPSAECs A549 A549, HeLa Huh7 Huh7 Huh7
Virus Respiratory viruses SARS- CoV-2 FCoV MERS- CoV RSV RSV IAV (H1N1 and H3N2) IAV IAV (H1N1 PR8) Hepatotrophic viruses HCV HCV HCV
International Journal of Bioprinting (2020)–Volume 6, Issue 4 15