Page 348 - IJB-10-6
P. 348
International Journal of Bioprinting Collagen hydrolysate-loaded ODMA/PEGDMA scaffold
Tetrahydrofuran, hydrochloric acid, ethyl acetate, hexane, sodium borate/sodium bicarbonate solution at a weight
acetonitrile, and trifluoroacetic acid were supplied by ratio of 5:2 in 200 mL of deionized water under nitrogen
QReC (New Zealand). Biological materials, such as CH gas. Methacrylic anhydride tetrahydrofuran was added
extracted from tuna tendon, were sourced from Chiang slowly to the top of the solution at 0.2 mL per 150 mL.
19
Mai University. Sterilization equipment included an Sodium hydroxide was added to maintain the pH above 8
ethylene oxide (EtO) sterilizer from Namwiwat Company during the reaction. Subsequently, 1 M hydrochloric acid
in Thailand, a gamma irradiation carrier type model JS 8900 was added to decrease the pH of the mixture to 2 when
IR-155 designed by Nordion International Inc. (Canada), dopamine methacrylate (DMA) was precipitated. Next,
and a beta radiation electron beam accelerator model MB ethyl acetate was used to extract the DMA, which was
20-16 manufactured by Mevex Corporation Ltd. (Canada). then recrystallized by adding hexane and dried using a
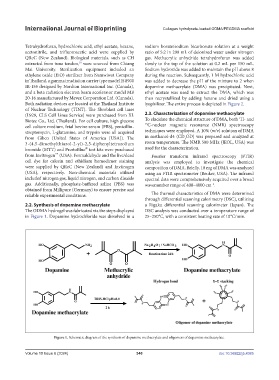
Both radiation devices are located at the Thailand Institute lyophilizer. The entire process is depicted in Figure 2.
of Nuclear Technology (TINT). The fibroblast cell lines
(L929, CLS Cell Lines Service) were purchased from XL 2.3. Characterization of dopamine methacrylate
1
Biotec Co., Ltd. (Thailand). For cell culture, high glucose To elucidate the chemical structure of DMA, both H- and
cell culture medium, fetal bovine serum (FBS), penicillin- 13 C-nuclear magnetic resonance (NMR) spectroscopy
streptomycin, L-glutamine, and trypsin were all acquired techniques were employed. A 10% (w/v) solution of DMA
from Gibco (United States of America [USA]). The in methanol-d4 (CD OD) was prepared and analyzed at
3
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium room temperature. The NMR 500 MHz (JEOL, USA) was
®
bromide (MTT) and PrestoBlue test kits were purchased used for the characterization.
™
from Invitrogen (USA). Formaldehyde and the live/dead Fourier transform infrared spectroscopy (FTIR)
cell dye for calcein and ethidium homodimer staining analysis was employed to investigate the chemical
were supplied by QReC (New Zealand) and Invitrogen composition of DMA. Briefly, 10 mg of DMA was analyzed
(USA), respectively. Non-chemical materials utilized using an FTIR spectrometer (Bruker, USA). The infrared
included nitrogen gas, liquid nitrogen, and carbon dioxide spectral data were comprehensively acquired over a broad
gas. Additionally, phosphate-buffered saline (PBS) was wavenumber range of 400–4000 cm .
−1
obtained from Millipore (Germany) to ensure precise and
reliable experimental conditions. The thermal characteristics of DMA were determined
through differential scanning calorimetry (DSC), utilizing
2.2. Synthesis of dopamine methacrylate a Rigaku differential scanning calorimeter (Japan). The
The ODMA hydrogel was fabricated via the steps displayed DSC analysis was conducted over a temperature range of
in Figure 1. Dopamine hydrochloride was dissolved in a 25–250°C, with a consistent heating rate of 10°C/min.
Figure 1. Schematic diagram of the synthesis of dopamine methacrylate and oligomers of dopamine methacrylate.
Volume 10 Issue 6 (2024) 340 doi: 10.36922/ijb.4385