Page 510 - IJB-10-6
P. 510
International Journal of Bioprinting Redefined collagen inks in cartilage printing
was observed for a 0.5% hydrogel, comparable to values concentration from 6 to 12 and 18 mM in the presence
in the literature. However, about 3 min was required of 1 M NaCl (data not shown). 39,40 A key insight from this
38
for a hydrogel with a thickness of 1 mm (i.e., the distance study is that fibril formation is expedited at lower ionic
between the rheometer plates) to reach Gʹ saturation at strengths due to the minimization of salting-out effects and
18
37°C (Figure 1a). Although this is considered too long for enhanced electrostatic interactions among amino acids.
high-precision extrusion bioprinting, extruded filaments We observed it by decreasing the concentration of NaCl
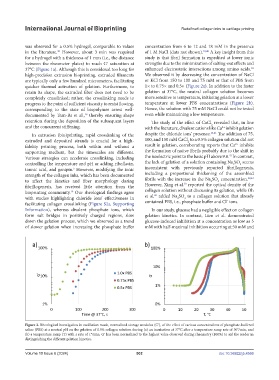
are typically only a few hundred micrometers, facilitating or KCl from 150 to 100 and 75 mM or that of PBS from
quicker thermal activation of gelation. Furthermore, to 1× to 0.75× and 0.5× (Figure 2a). In addition to the faster
retain its shape, the extruded fiber does not need to be gelation at 37°C, the neutral collagen solution becomes
completely crosslinked; rather, the crosslinking needs to more sensitive to temperature, initiating gelation at a lower
progress to the point of sufficient viscosity to resist flowing, temperature at lower PBS concentrations (Figure 2b).
corresponding to the state of biopolymer arrest well- Hence, the solution with 75 mM NaCl could not be tested
documented by Tran-Ba et al., thereby ensuring shape even while maintaining a low temperature.
26
retention during the deposition of the subsequent layers The study of the effect of CaCl revealed that, in line
2
and the concurrent stiffening. with the literature, divalent cations like Ca inhibit gelation
2+
In extrusion (bio)printing, rapid crosslinking of the despite the chloride ions’ presence. 15,41 The addition of 75,
extruded and deposited strands is crucial for a high- 100, and 150 mM CaCl to a 0.5% collagen solution did not
2
2+
fidelity printing process, both within and without a result in gelation, corroborating reports that Ca inhibits
supporting medium, but the timescales are different. the formation of native fibrils probably due to the shift in
42
Various strategies can accelerate crosslinking, including the isoelectric point to the basic pH above 9.0. In contrast,
controlling the temperature and pH or adding riboflavin, the lack of gelation of a solution containing Na SO seems
2
4
tannic acid, and genipin. However, modifying the ionic inconsistent with previously reported fibrillogenesis,
5
strength of the collagen inks, which has been documented including a proportional thickening of the assembled
20,43
to affect the kinetics and fiber morphology during fibrils with the increase in the Na SO concentration.
2
4
20
fibrillogenesis, has received little attention from the However, Xing et al. reported the optical density of the
bioprinting community. Our rheological findings agree collagen solution without discussing its gelation, while Oh
19
43
with studies highlighting chloride ions’ effectiveness in et al. added Na SO to a collagen solution that already
4
2
−
facilitating collagen crosslinking (Figure S2a, Supporting contained PBS, i.e., phosphate buffer and Cl ions.
Information), whereas divalent phosphate ions, which In our study, glucose had a negligible effect on collagen
form salt bridges in positively charged regions, slow gelation kinetics. In contrast, Lien et al. demonstrated
down the gelation process, which we observed as a trend glucose-induced inhibition at a concentration as low as 5
of slower gelation when increasing the phosphate buffer mM with half-maximal inhibition occurring at 50 mM and
Figure 2. Rheological investigation in oscillation mode, normalized storage modulus (Gʹ), of the effect of various concentrations of phosphate-buffered
saline (PBS) at a neutral pH on the gelation of 0.5% collagen solution during (a) an incubation at 37°C after a temperature ramp rate of 30°/min, and
(b) a temperature ramp (T) with a rate of 1°/min. Gʹ has been normalized to the highest value observed during rheometry (100%) to aid the reader in
distinguishing the different gelation kinetics.
Volume 10 Issue 6 (2024) 502 doi: 10.36922/ijb.4566