Page 74 - IJB-7-2
P. 74
A Scientometric Analysis
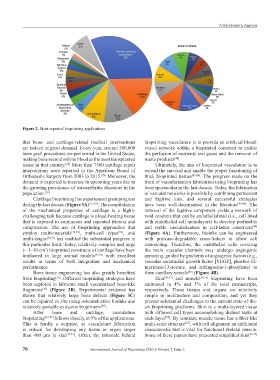
Figure 2. Most reported bioprinting applications.
that bone- and cartilage-related medical interventions bioprinting vasculature is to provide an artificial blood-
are indeed in great demand. Every year, around 500,000 vessel network within a bioprinted construct to enable
bone graft procedures are performed in the United States, the perfusion of nutrients and gases and the removal of
making bone second only to blood as the most transplanted waste products .
[44]
tissue in that country. More than 7500 cartilage repair Ultimately, the aim of bioprinted vasculature is to
[28]
interventions were reported to the American Board of extend the survival and enable the proper functioning of
Orthopedic Surgery from 2003 to 2015. Moreover, the thick bioprinted tissues [45,46] . The progress made on the
[29]
demand is expected to increase in upcoming years due to front of vascularization fabrication using bioprinting has
the growing prevalence of osteoarthritis disorders in the been spectacular in the last decade. Today, the fabrication
population. [30] of vascular networks is possible by combining permanent
Cartilage bioprinting has experienced great progress and fugitive inks, and several successful strategies
during the last decade (Figure 3A) [31,32] . The recapitulation have been well-documented in the literature [47,48] . The
of the mechanical properties of cartilage is a highly removal of the fugitive component yields a network of
challenging task because cartilage is a load-bearing tissue void conduits that can be endothelialized (i.e., cell-lined
that is exposed to continuous and repeated friction and with endothelial cell monolayers) to develop perfusable
compression. The use of bioprinting approaches that and stable vascularization in cell-laden constructs
[48]
employ multi-materials [33,34] , multi-cell types , and (Figure 4A). Furthermore, bioinks can be engineered
[31]
multi-stages [34,35] has enabled the substantial progress in with protease-degradable cross-linkers to allow cell
this particular front. Today, relatively complex and large remodeling. Therefore, the endothelial cells covering
(~ 1–10 cm ) bioprinted constructs of cartilage have been the main vascular channels may undergo angiogenic
3
implanted in large animal models [33,35] with excellent sprouting, guided by gradients of angiogenic factors (e.g.,
results in terms of both integration and mechanical vascular endothelial growth factor [VEGF], phorbol-12-
performance. myristate-13-acetate, and sphingosine-1-phosphate) to
Bone tissue engineering has also greatly benefited form capillary vessels (Figure 4B).
[49]
from bioprinting . Different bioprinting strategies have Skin [50,51] and muscle [52,53] bioprinting have been
[36]
been explored to fabricate small vascularized bone-like addressed in 4% and 3% of the total manuscripts,
fragments (Figure 3B). Experimental evidence has respectively. These tissues and organs are relatively
[37]
shown that relatively large bone defects (Figure 3C) simple in architecture and composition, and yet they
can be repaired in situ using osteoinductive bioinks and present substantial challenges to the current state-of-the-
relatively portable extrusion bioprinters . art bioprinting platforms. Skin is a multi-layered tissue
[38]
After bone and cartilage, vasculature with different cell types accomplishing distinct tasks at
bioprinting [39-41] follows closely, at 9% of the applications. each layer . By contrast, muscle tissue has a fiber-like
[54]
This is hardly a surprise, as vasculature fabrication multi-scale structure , with cell alignment an additional
[55]
is critical for developing any tissue or organ larger characteristic that is vital for functional skeletal muscle.
than 400 µm in size [42,43] . Often, the rationale behind Some of these papers have presented simplified skin [56-58]
70 International Journal of Bioprinting (2021)–Volume 7, Issue 2