Page 75 - IJB-7-2
P. 75
González, et al.
A
B C
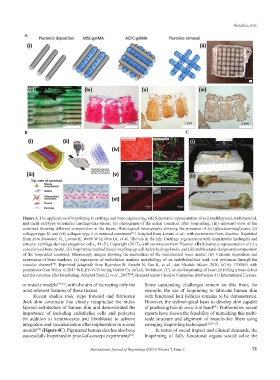
Figure 3. The application of bioprinting in cartilage and bone engineering. (A) Schematic representation of a (i) multilayered, multimaterial,
and multi-cell-type bioprinted cartilage-like tissue; (ii) photograph of the actual construct after bioprinting; (iii) sideward view of the
construct showing different composition in the layers. Histological micrographs showing the presence of (iv) glycosaminoglycans, (v)
collagen type II, and (vi) collagen type 1 in matured constructs . Adapted from Levato et al., with permission from Elsevier. Reprinted
[31]
from Acta Biomater, 61, Levato R, Webb W R, Otto IA, et al., The bio in the ink: Cartilage regeneration with bioprintable hydrogels and
articular cartilage-derived progenitor cells., 41–53, Copyright (2017), with permission from Elsevier. (B) Schematic representation of (i) a
vascularized bone model, (ii) bioprinting method based on piling-up cell-laden hydrogel rods, and (iii) architectural design and composition
of the bioprinted construct. Microscopy images showing the maturation of the vascularized bone model: (iv) Calcium deposition and
expression of bone markers; (v) expression of endothelial markers morphology of an endothelial-like wall; (vi) perfusion through the
vascular channel . Reprinted (adapted) from Byambaa B, Annabi N, Yue K, et al., Adv Healthc Mater, 2020, 6(16): 1700015 with
[37]
permission from Wiley. © 2017 WILEY-VCH Verlag GmbH Co. KGaA, Weinheim. (C). In situ bioprinting of bone: (i) Filling a bone defect
and (ii) outcome after bioprinting. Adapted from Li et al., 2017 ; licensed under Creative Commons Attribution 4.0 International License.
[38]
or muscle models [59-61] , with the aim of recreating only the Some outstanding challenges remain on this front; for
most relevant features of these tissues. example, the use of bioprinting to fabricate human skin
Recent studies took steps forward and fabricated with functional hair follicles remains to be demonstrated.
thick skin constructs that closely recapitulate the multi- However, the technological basis to develop skin capable
layered architecture of human skin and demonstrated the of producing hair de novo is at hand . Furthermore, recent
[64]
importance of including endothelial cells and pericytes reports have shown the feasibility of mimicking this multi-
(in addition to keratinocytes and fibroblasts) to achieve scale structure and alignment of muscle-like fibers using
integration and vascularization after implantation in animal emerging bioprinting techniques [12,65-67] .
models (Figure 4C). Pigmented human skin has also been In terms of social impact and clinical demands, the
[62]
successfully bioprinted in proof-of-concept experiments . bioprinting of fully functional organs would solve the
[63]
International Journal of Bioprinting (2021)–Volume 7, Issue 2 71