Page 136 - IJB-7-3
P. 136
Multiresponsive Graphene-Oxide Embedded ECM Hydrogel for 3D Bioprinting
lead to a superior exfoliation level within the hydrogel methods, such as long periods of sonication [58,59] , which
(Figure 3A). Protein adsorption on GO after exposure can alter protein structure within bioactive hydrogels .
[60]
to culture media was confirmed by a 23.57 ± 3.51%
decrease in medium protein concentration, as observed in 3.3. Characterization of SISMA-GO hydrogel
Figure 3B. This corresponds to approximately 2.29 µg of crosslinking
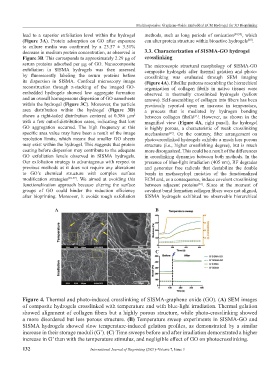
serum proteins adsorbed per µg of GO. Nanocomposite The microscopic structural morphology of SISMA-GO
exfoliation in SISMA hydrogels was then assessed composite hydrogels after thermal gelation and photo-
by fluorescently labeling the serum proteins before crosslinking was evaluated through SEM imaging
its dispersion in SISMA. Confocal microscopy image (Figure 4A). Fibrillar patterns resembling the hierarchical
reconstruction through z-stacking of the imaged GO- organization of collagen fibrils in native tissues were
embedded hydrogels showed low aggregate formation observed in thermally crosslinked hydrogels (yellow
and an overall homogeneous dispersion of GO nanosheets arrows). Self-assembling of collagen into fibers has been
within the hydrogel (Figure 3C). Moreover, the particle previously reported upon an increase in temperature,
area distribution within the hydrogel (Figure 3D) a process that is mediated by hydrogen bonding
shows a right-tailed distribution centered at 0.386 µm 2 between collagen fibrils . However, as shown in the
[61]
with a few out-of-distribution cases, indicating that low magnified view (Figure 4A, right panel), the hydrogel
GO aggregation occurred. The high frequency at this is highly porous, a characteristic of weak crosslinking
specific area value may have been a result of the image mechanisms . On the contrary, fiber arrangement on
[62]
resolution limits, which means that smaller GO sheets photo-crosslinked hydrogels exhibits a much less porous
may exist within the hydrogel. This suggests that protein structure (i.e., higher crosslinking degree), but is much
coating before dispersion may contribute to the adequate more disorganized. This could be a result of the differences
GO exfoliation levels observed in SISMA hydrogels. in crosslinking dynamics between both methods. In the
Our exfoliation strategy is advantageous with respect to presence of blue-light irradiation (405 nm), RF degrades
previous methods as it does not require any alterations and generates free radicals that destabilize the double
to GO’s chemical structure with complex surface bonds in methacryloyl moieties of the functionalized
modification strategies [55-57] . We aimed at avoiding this ECM and, as a consequence, induce covalent crosslinking
functionalization approach because altering the surface between adjacent proteins . Since at the moment of
[63]
groups of GO could hinder the reduction efficiency covalent bond formation collagen fibers were not aligned,
after bioprinting. Moreover, it avoids rough exfoliation SISMA hydrogels exhibited no observable hierarchical
A B
C
Figure 4. Thermal and photo-induced crosslinking of SISMA-graphene oxide (GO). (A) SEM images
of composite hydrogels crosslinked with temperature and with blue-light irradiation. Thermal gelation
showed alignment of collagen fibers but a highly porous structure, while photo-crosslinking showed
a more disordered but less porous structure. (B) Temperature sweep experiments in SISMA-GO and
SISMA hydrogels showed slow temperature-induced gelation profiles, as demonstrated by a similar
increase in their storage moduli (G’). (C) Time sweeps before and after irradiation demonstrated a higher
increase in G’ than with the temperature stimulus, and negligible effect of GO on photocrosslinking.
132 International Journal of Bioprinting (2021)–Volume 7, Issue 3