Page 119 - IJB-8-1
P. 119
Lou, et al.
release of nickel ions. This explains why the antibacterial following the same process as that for WNC and PNC.
activity of PNC is better than that of WNC because On the other hand, electron transfer occurs between GNC
PNC can produce a higher concentration of nickel ions and bacteria, inhibiting or even interrupting the metabolic
[65]
around it. GNC and PNC have similar morphological process of the bacteria . The electrons accepted by
characteristics, but the former released fewer nickel ions GO mediate the generation of ROS on its oxygen-
than the latter. This is mainly because the presence of GO containing functional group , and some of the ROS
[66]
on the surface of GNC hinders the release of nickel ions. will become intracellular ROS due to the internalization
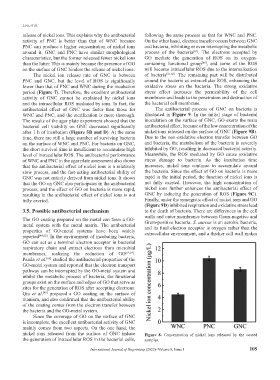
The nickel ion release rate of GNC is between of bacteria [67,68] . The remaining part will be distributed
PNC and GNC, but the level of ROS is significantly around the bacteria as extracellular ROS, enhancing the
lower than that of PNC and WNC during the incubation oxidative stress on the bacteria. The strong oxidative
period (Figure 7). Therefore, the excellent antibacterial stress effect increases the permeability of the cell
activity of GNC cannot be explained by nickel ions membrane and leads to the penetration and destruction of
and the intracellular ROS mediated by ions. In fact, the the bacterial cell membrane.
antibacterial effect of GNC was faster than those for The antibacterial process of GNC on bacteria is
WNC and PNC, and the sterilization is more thorough. illustrated in Figure 9. In the initial stage of bacterial
The results of the agar plate experiment showed that the inoculation on the surface of GNC, GO exerts the main
bacterial cell viability on GNC decreased significantly antibacterial effect, because of the low concentration of the
after 1 h of incubation (Figure 5B and D). At the same nickel ions released on the surface of GNC (Figure 9B).
time, there are still a large number of surviving bacteria Due to the non-oxidative electron transfer between GO
on the surface of WNC and PNC. For bacteria on GNC, and bacteria, the metabolism of the bacteria is severely
the short survival time is insufficient to accumulate high inhibited by GO, resulting in decreased bacterial activity.
level of intracellular ROS. The antibacterial performance Meanwhile, the ROS mediated by GO cause oxidative
of WNC and PNC in the agar plate assessment also shows stress damage to bacteria. As the incubation time
that the antibacterial effect of nickel ions is a relatively increases, nickel ions continue to accumulate around
slow process, and the fast-acting antibacterial ability of the bacteria. Since the effect of GO on bacteria is more
GNC was not entirely derived from nickel ions. It shows rapid in the initial period, the function of nickel ions is
that the GO on GNC also participates in the antibacterial not fully exerted. However, the high concentration of
process, and the effect of GO on bacteria is more rapid, nickel ions further enhances the antibacterial effect of
resulting in the antibacterial effect of nickel ions is not GNC by inducing the generation of ROS (Figure 9C).
fully exerted. Finally, under the synergistic effect of nickel ions and GO
(Figure 9D) inhibited respiration and oxidative stress lead
3.5. Possible antibacterial mechanism to the death of bacteria. There are differences in the cell
walls and outer membranes between Gram-negative and
The GO coating prepared on the metal can form a GO- Gram-positive bacteria. S. aureus is an aerobic bacteria,
metal system with the metal matrix. The antibacterial and its final electron acceptor is oxygen rather than the
properties of GO-metal systems have been widely extracellular environment, and a thicker cell wall makes
reported [44,62] . In the environment of incubating bacteria,
GO can act as a terminal electron acceptor in bacterial
respiratory chain and extract electrons from microbial
membranes, realizing the reduction of GO [63,64] .
Panda et al. studied the antibacterial properties of the
[65]
GO-metal system and reported that the electron transport
pathway can be interrupted by the GO-metal system and
inhibit the metabolic process of bacteria, the functional
groups exist on the surface and edges of GO that serve as
sites for the generation of ROS after accepting electrons.
Qiu et al. prepared a GO coating on the surface of
[49]
titanium, and also confirmed that the antibacterial ability
of the coating comes from the electron transfer between
the bacteria and the GO-metal system.
Since the coverage of GO on the surface of GNC
is incomplete, the excellent antibacterial activity of GNC
mainly comes from two aspects. On the one hand, the
nickel ions released from the surface of GNC induce Figure 8. Concentration of nickel ions released by the coated
the generation of intracellular ROS in the bacterial cells, samples.
International Journal of Bioprinting (2022)–Volume 8, Issue 1 105