Page 75 - IJB-8-1
P. 75
Seiti, et al.
(2) Cell adhesion and viability on PEDOT: PSS samples be also a major (co-)cause of these results. At 48 h, a
As shown in Figure 6I and J, the immunofluorescence detectable presence of cellular agglomerates was visible
assays of NSCs cultured on Matrigel-coated AJ P near the well edges (Figure 7B). Figure 7C shows the
®
PEDOT: PSS printed samples show limited cellular findings of the cell viability rATP assay (time points 24 h,
adhesion and no detectable proliferation subsequently. 48 h, and 96 h) of NSCs cultured on Matrigel-coated
®
This is in contrast with the data on NSCs seeded on AJ P printed PEDOT: PSS samples. At time point 24 h,
Matrigel-coated plastic control samples, which exhibit NSCs showed a healthier state on the plastic control than
a healthy state condition of cellular adhesion and on the sample. At 48 h, the metabolic activity of the NSCs
proliferation along with neural networks formation. on the substrates further reduced. Eventually, at 96 h, the
This can be caused by a detected hydrophobic behavior cytotoxicity of the PEDOT: PSS samples was ×10 higher
of the samples when in contact with the NSCs/medium than the plastic controls. This could be related to the
culture dispersion. When poured on the samples, the residual presence of the co-solvent DEG in the printed
NSC dispersion remained confined into a drop-like pattern, which does not fully evaporate after sintering. To
shape, limiting cell spreading on the surface (Figure 7A). validate this hypothesis, an indirect cell viability assay
A potential cytotoxicity of the used PEDOT: PSS ink can was performed on printed PEDOT: PSS samples sintered
A C D
B
E F
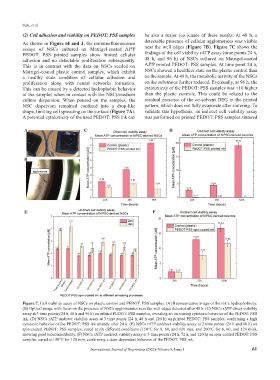
Figure 7. Cell viability assay of NSCs on plastic control and PEDOT: PSS samples. (A) Representative image of the ink’s hydrophobicity.
(B) Optical image with focus on the presence of NSCs agglomerates near the well edges detected after 48 h. (C) NSCs rATP direct viability
assay at 3 time points (24 h, 48 h and 96 h) on printed PEDOT: PSS samples, revealing an increasing cytotoxic behavior of the PEDOT: PSS
ink. (D) NSCs rATP undirect viability assay at 3 time points (24 h, 48 h and 120 h) on printed PEDOT: PSS samples, confirming a high
cytotoxic behavior of the PEDOT: PSS ink already after 24 h. (E) NSCs rATP undirect viability assay at 2 time points (24 h and 48 h) on
spin-coated PEDOT: PSS samples, cured at six different conditions (150°C for 8, 60, and 120 min, and 200°C for 8, 60, and 120 min),
showing good biocompatibility. (F) NSCs rATP undirect viability assay at 3 time points (24 h, 72 h, and 120 h) on spin-coated PEDOT: PSS
samples, cured at 150°C for 120 min, confirming a dose-dependent behavior of the PEDOT: PSS ink.
International Journal of Bioprinting (2022)–Volume 8, Issue 1 61