Page 74 - IJB-8-1
P. 74
AJ P of Bioelectrical Devices
®
profile of one deposition is reflected and cumulates with A B
the successive one, leading to remarkable inaccuracy and
inefficient build thickness.
Accordingly, a layer-by-layer print strategy
of (thin) adjacent overlapped lines is typically
recommended in PE to print well defined and solid
lines. It is also to note that ink composition for 3D AJ P
®
micro-structuring is still under investigation within the C D
research community but getting increasing interest.
Eventually, the electrodes on the Parylene-C-coated
NTE substrates have the lowest electrical resistance,
given the highly insulating properties of the coating.
This is beneficial for the reduction of the dispersion of
the electrical signal.
3.2. Biocompatibility and cellular adhesion E F
(1) Immunofluorescence of the seeded Parylene-C-
coated NTE substrates
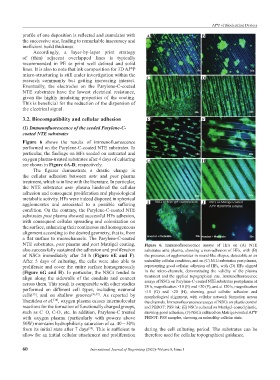
Figure 6 shows the results of immunofluorescence
performed on the Parylene-C-coated NTE substrates. In
particular, the findings on HFs seeded on untreated and
oxygen plasma-treated substrates after 4 days of culturing G H
are shown in Figure 6A‑D, respectively.
The figures demonstrate a drastic change in
the cellular adhesion between ante and post plasma
treatment, which is in line with the literature. In particular,
the NTE substrates ante plasma hindered the cellular
adhesion and consequent proliferation and physiological
metabolic activity. HFs were indeed disposed in spherical
agglomerates and associated to a possible suffering I J
condition. On the contrary, the Parylene-C-coated NTE
substrates post plasma showed successful HFs adhesion,
with consequent cellular spreading and colonization on
the surface, enhancing their continuous and homogeneous
alignment according to the desired geometry, that is, from
a flat surface to microchannels. The Parylene-C-coated
NTE substrates, post plasma and post Matrigel coating, Figure 6. Immunofluorescence assays of HFs on (A) NTE
also successfully sustained the adhesion and proliferation substrates ante plasma, showing a non-adhesion of HFs, with (B)
of NSCs immediately after 24 h (Figure 6E and F). the presence of agglomerates in round-like shapes, detectable as an
After 5 days of culturing, the cells were also able to unhealthy cellular condition, and on (C) NTE substrates post plasma,
proliferate and cover the entire surface homogeneously representing good cellular adhesion of HFs, with (D) HFs aligned
(Figure 6G and H). In particular, the NSCs tended to in the micro-channels, demonstrating the validity of the plasma
align along the sidewalls of the conduits and connect treatment and the applied topographical cue. Immunofluorescence
assays of NSCs on Parylene-C-coated NTE substrates post plasma at
across them. This result is comparable with other studies 24 h, magnification ×10 (E) and ×20 (F), and at 120 h, magnification
performed on different cell types, including neuronal ×10 (G) and ×20 (H), showing good cellular adhesion and
cells , and on shallow grooves [36,37] . As reported by morphological alignment, with cellular network formation across
[35]
Trantidou et al. , oxygen plasma causes intermolecular the channels. Immunofluorescence assays of NSCs on plastic control
[38]
reactions for the formation of functionally charged groups, and PEDOT: PSS ink: (E) NSCs cultured on Matrigel-coated plastic,
such as C=O, C-O, etc. In addition, Parylene-C treated showing good adhesion, (F) NSCs cultured on Matrigel-coated AJ P
®
with oxygen plasma (particularly with powers above PEDOT: PSS samples, showing an unhealthy cellular state.
50W) maintains hydrophilicity saturation of ca. 40 – 50%
from its initial state after 7 days . This is sufficient to during the cell culturing period. The substrates can be
[38]
allow for an initial cellular attachment and proliferation therefore used for cellular topographical guidance.
60 International Journal of Bioprinting (2022)–Volume 8, Issue 1