Page 105 - IJB-8-2
P. 105
Lee, et al.
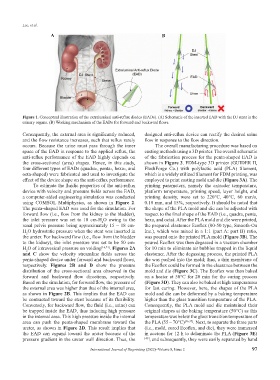
A B
Figure 1. Conceptual illustration of the extraluminal anti-reflux diodes (EADs). (A) Schematic of the inserted EAD with the DJ stent in the
urinary organs. (B) Working mechanism of the EADs for forward and backward flows.
Consequently, the external area is significantly reduced, designed anti-reflux device can rectify the desired urine
and the flow resistance increases, such that reflux rarely flow in response to the flow direction.
occurs. Because the urine must pass through the inner The overall manufacturing procedure was based on
space of the EAD in response to the applied reflux, the casting methods using a 3D printer. The overall schematic
anti-reflux performance of the EAD highly depends on of the fabrication process for the penta-shaped EAD is
the cross-sectional (area) shapes. Hence, in this study, shown in Figure 3. FDM-type 3D printer (GUIDER II,
four different types of EADs (quadra-, penta-, hexa-, and FlashForge Co.) with polylactic acid (PLA) filament,
octa-shaped) were fabricated and used to investigate the which is a widely utilized filament for FDM printing, was
effect of the device shape on the anti-reflux performance. employed to print casting mold and die (Figure 3A). The
To estimate the fluidic properties of the anti-reflux printing parameters, namely the extruder temperature,
device with velocity and pressure fields across the EAD, platform temperature, printing speed, layer height, and
a computer-aided engineering simulation was conducted printing density, were set to 220°C, 40°C, 60 mm/s,
using COMSOL Multiphysics, as shown in Figure 2. 0.18 mm, and 15%, respectively. It should be noted that
The penta-shaped EAD was used for the simulation. For the shape of the PLA mold and die can be adjusted with
forward flow (i.e., flow from the kidney to the bladder), respect to the final shape of the EAD (i.e., quadra, penta,
the inlet pressure was set to 18 cm-H O owing to the hexa, and octa). After the PLA mold and die were printed,
2
renal pelvis pressure being approximately 15 – 18 cm- the prepared elastomer Ecoflex (00-50 type, Smooth-On
H O hydrostatic pressure when the stent was inserted in Inc.), which was mixed in a 1:1 (part A: part B) ratio,
2
the ureter. For backward flow (i.e., flow from the bladder was poured onto the printed PLA mold (Figure 3B). The
to the kidney), the inlet pressure was set to be 50 cm- poured Ecoflex was then degassed in a vacuum chamber
H O of intravesical pressure on voiding [16,21] . Figures 2A for 10 min to eliminate air bubbles trapped in the liquid
2
and C show the velocity streamline fields across the elastomer. After the degassing process, the printed PLA
penta-shaped device under forward and backward flows, die was pushed into the mold; thus, a thin membrane of
respectively. Figures 2B and D show the pressure the Ecoflex could be formed in the clearance between the
distribution of the cross-sectional area observed in the mold and die (Figure 3C). The Ecoflex was then baked
forward and backward flow directions, respectively. on a heater at 50°C for 20 min for the curing process
Based on the simulation, for forward flow, the pressure of (Figure 3D). They can also be baked at high temperatures
the external area was higher than that of the internal area, for fast curing. However, here, the shapes of the PLA
as shown in Figure 2B. This implies that the EAD can mold and die can be deformed by a baking temperature
be contracted toward the stent because of its flexibility. higher than the glass transition temperature of the PLA.
Conversely, for backward flow, the fluid (i.e., urine) can Consequently, the PLA mold and die maintained their
be trapped inside the EAD, thus inducing high pressure original shapes at the baking temperature (50°C) as this
in the internal area. This high pressure inside the internal temperature was below the glass transition temperature of
area can push the penta-shaped membrane toward the the PLA (55 – 70°C) [46,47] . Next, to separate the three parts
ureter, as shown in Figure 2D. This result implies that (i.e., mold, cured Ecoflex, and die), they were immersed
the EAD can expand toward the ureter because of the in acetone for 12 h to delaminate the PLA (Figure 3E)
pressure gradient in the ureter wall direction. Thus, the [48] , and subsequently, they were easily separated by hand
International Journal of Bioprinting (2022)–Volume 8, Issue 2 97