Page 24 - IJB-8-2
P. 24
Bioprinting Gelatin-Recombinant Type III Collagen Hydrogel for Wound Healing
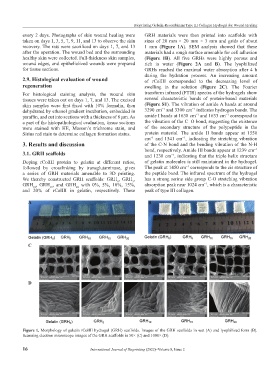
every 2 days. Photographs of skin wound healing were GRH materials were then printed into scaffolds with
taken on days 1, 3, 5, 7, 9, 11, and 13 to observe the skin sizes of 20 mm × 20 mm × 3 mm and grids of about
recovery. The rats were sacrificed on days 1, 7, and 13 1 mm (Figure 1A). SEM analysis showed that these
after the operation. The wound bed and the surrounding materials had a rough surface amenable for cell adhesion
healthy skin were collected. Full-thickness skin samples, (Figure 1B). All five GRHs were highly porous and
wound edges, and epithelialized wounds were prepared rich in water (Figure 2A and B). The lyophilized
for tissue sections. GRHs reached the maximal water absorption after 4 h
during the hydration process. An increasing amount
2.9. Histological evaluation of wound of rColIII corresponded to the decreasing level of
regeneration swelling in the solution (Figure 2C). The Fourier
For histological staining analysis, the wound skin transform infrared (FTIR) spectra of the hydrogels show
tissues were taken out on days 1, 7, and 13. The excised several characteristic bands of protein-based materials
skin samples were first fixed with 10% formalin, then (Figure S1). The vibration of amide A bands at around
−1
−1
dehydrated by ethanol gradient incubation, embedded in 3290 cm and 3300 cm indicates hydrogen bonds. The
−1
−1
paraffin, and cut into sections with a thickness of 8 μm. As amide I bands at 1638 cm and 1633 cm correspond to
a part of the histopathological evaluation, tissue sections the vibration of the C=O bond, suggesting the existence
were stained with HE, Masson’s trichrome stain, and of the secondary structure of the polypeptide in the
Sirius red stain to determine collagen formation status. protein material. The amide II bands appear at 1550
cm and 1541 cm , indicating the stretching vibration
−1
−1
3. Results and discussion of the C-N bond and the bending vibration of the N-H
bond, respectively. Amide III bands appear at 1239 cm
−1
3.1. GRH scaffolds and 1238 cm , indicating that the triple helix structure
−1
Doping rColIII protein to gelatin at different ratios, of gelatin molecules is still maintained in the hydrogel.
−1
followed by crosslinking by transglutaminase, gives The peak at 1450 cm corresponds to the cis structure of
a series of GRH materials amenable to 3D printing. the peptide bond. The infrared spectrum of the hydrogel
We thereby constructed GRH scaffolds: GRH , GRH , has a strong serine side group C-O stretching vibration
5
0
GRH , GRH , and GRH with 0%, 5%, 10%, 15%, absorption peak near 1024 cm , which is a characteristic
−1
10
20
15
and 20% of rColIII in gelatin, respectively. These peak of type III collagen.
A B
C
D
Figure 1. Morphology of gelatin rColIII hydrogel (GRH) scaffolds. Images of the GRH scaffolds in wet (A) and lyophilized form (B).
Scanning electron microscope images of the GRH scaffolds in 50× (C) and 1000× (D).
16 International Journal of Bioprinting (2022)–Volume 8, Issue 2