Page 113 - IJB-8-3
P. 113
Yang, et al.
A
B
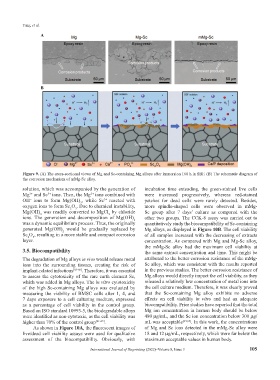
Figure 9. (A) The cross-sectional views of Mg and Sc-containing Mg alloys after immersion 168 h in SBF. (B) The schematic diagram of
the corrosion mechanism of mMg-Sc alloy.
solution, which was accompanied by the generation of incubation time extending, the green-stained live cells
Mg and Sc ions. Then, the Mg ions combined with were increased progressively, whereas red-stained
3+
2+
2+
OH ions to form Mg(OH) , while Sc reacted with patches for dead cells were rarely detected. Besides,
3+
−
2
oxygen ions to form Sc O . Due to chemical instability, more spindle-shaped cells were observed in mMg-
2
3
Mg(OH) was readily converted to MgCl by chloride Sc group after 7 days’ culture as compared with the
2
2
ions. The generation and decomposition of Mg(OH) other two groups. The CCK-8 assay was carried out to
2
was a dynamic equilibrium process. Thus, the originally quantitatively study the biocompatibility of Sc-containing
generated Mg(OH) would be gradually replaced by Mg alloys, as displayed in Figure 10B. The cell viability
2
Sc O , resulting in a more stable and compact corrosion of all samples increased with the decreasing of extracts
2
3
layer. concentration. As compared with Mg and Mg-Sc alloy,
the mMg-Sc alloy had the maximum cell viability at
3.5. Biocompatibility the same extract concentration and time. This might be
The degradation of Mg alloys in vivo would release metal attributed to the better corrosion resistance of the mMg-
ions into the surrounding tissues, creating the risk of Sc alloy, which was consistent with the results reported
implant-related infections [58-60] . Therefore, it was essential in the previous studies. The better corrosion resistance of
to assess the cytotoxicity of the rare earth element Sc, Mg alloys would directly impact the cell viability, as they
which was added in Mg alloys. The in vitro cytotoxicity released a relatively low concentration of metal ions into
of the high Sc-containing Mg alloys was evaluated by the cell culture medium. Therefore, it was clearly proved
measuring the viability of BMSC cells after 1, 4, and that the Sc-containing Mg alloy exhibits no adverse
7 days exposure to a cell culturing medium, expressed effects on cell viability in vitro and had an adequate
as a percentage of cell viability in the control group. biocompatibility. Prior studies have reported that the total
Based on ISO standard 10993-5, the biodegradable alloys Mg ion concentration in human body should be below
were identified as non-cytotoxic, as the cell viability was 480 μg/mL, and the Sc ion concentration below 300 μg/
higher than 70% of the control group [61-63] . mL was acceptable [64,65] . In this work, the concentrations
As shown in Figure 10A, the fluorescent images of of Mg and Sc ions detected in the mMg-Sc alloy were
live/dead cell viability assays were used for qualitative 18 and 12 μg/mL, respectively, which were far below the
assessment of the biocompatibility. Obviously, with maximum acceptable values in human body.
International Journal of Bioprinting (2022)–Volume 8, Issue 3 105