Page 154 - IJB-8-3
P. 154
3D-bioprinted HERS-DPCs for Alveolar Bone Regeneration
A B
C
F
D E
G
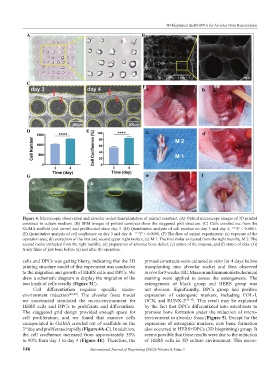
Figure 4. Microscopic observation and alveolar socket transplantation of printed construct. (A) Optical microscope images of 3D printed
construct in culture medium. (B) SEM images of printed construct show the staggered grid structure. (C) Cells crawled out from the
GelMA scaffold (red arrow) and proliferated since day 3. (D) Quantitative analysis of cell number on day 3 and day 4, ∗∗∗∗ P < 0.0001.
(E) Quantitative analysis of cell confluence on day 3 and day 4, ∗∗∗∗ P < 0.0001. (F) The flow of animal experiments: (a) exposure of the
operation area; (b) extraction of the first and second upper right molars; (c) M 1: The first molar extracted from the right maxilla, M 2: The
second molar extracted from the right maxilla; (d) preparation of alveolar bone defect; (e) suture of the mucosa; and (f) suture of skin. (G)
X-ray films of jaw bone before (a) and after (b) operation.
cells and DPCs was getting blurry, indicating that the 3D printed constructs were cultured in vitro for 4 days before
printing structure model of this experiment was conducive transplanting into alveolar socket and then observed
to the migration and growth of HERS cells and DPCs. We in vivo for 8 weeks. HE, Masson and immunohistochemical
drew a schematic diagram to display the migration of the staining were applied to assess the osteogenesis. The
two kinds of cells vividly (Figure 3C). osteogenesis of blank group and HERS group was
Cell differentiation requires specific micro- not obvious. Significantly, DPCs group had positive
environment induction [42-44] . The alveolar fossa model expression of osteogenic markers, including COL-I,
we constructed simulated the micro-environment for OCN, and RUNX-2 [45-47] . This result may be explained
HERS cells and DPCs to proliferate and differentiate. by the fact that DPCs differentiated into osteoblasts to
The staggered grid design provided enough space for promote bone formation under the induction of micro-
cell proliferation, and we found that massive cells environment in alveolar fossa (Figure 5). Except for the
encapsulated in GelMA crawled out of scaffolds on the expression of osteogenic markers, new bone formation
3 day and proliferated rapidly (Figure 4A-C). In addition, also occurred in HERS+DPCs (3D bioprinting) group. It
rd
the cell confluence increased from approximately 55% seems possible that these results were due to the induction
to 80% from day 3 to day 4 (Figure 4E). Therefore, the of HERS cells in 3D culture environment. This micro-
146 International Journal of Bioprinting (2022)–Volume 8, Issue 3