Page 150 - IJB-8-3
P. 150
3D-bioprinted HERS-DPCs for Alveolar Bone Regeneration
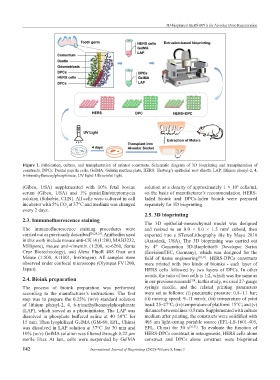
Figure 1. Fabrication, culture, and transplantation of printed constructs. Schematic diagram of 3D bioprinting and transplantation of
constructs. DPCs: Dental papilla cells, GelMA: Gelatin methacrylate, HERS: Hertwig’s epithelial root sheath, LAP: lithium phenyl-2, 4,
6-trimethylbenzoylphosphinate, UV light: Ultraviolet light.
(Gibco, USA) supplemented with 10% fetal bovine solution at a density of approximately 1 × 10 cells/mL
6
serum (Gibco, USA) and 1% penicillin/streptomycin on the basis of manufacturer’s recommendation. HERS-
solution (Solarbio, CHN). All cells were cultured in cell laded bioink and DPCs-laden bioink were prepared
incubator with 5% CO at 37°C and medium was changed separately for 3D bioprinting.
2
every 2 days.
2.5. 3D bioprinting
2.3. Immunofluorescence staining
The 3D epithelial-mesenchymal model was designed
The immunofluorescence staining procedures were and resized to an 8.0 × 8.0 × 1.5 mm cuboid, then
3
carried out as previously described [26,28,29] . Antibodies used exported into a STereoLithography file by Maya 2016
in this work include mouse anti-CK14 (1:200, MAB3232, (Autodesk, USA). The 3D bioprinting was carried out
Millipore), mouse anti-vimentin (1:200, sc-6260, Santa by 4 Generation 3D-Bioplotter® Developer Series
th
Cruz Biotechnology), and Alexa FluoR 488 Goat anti (EnvisionTEC, Germany), which was designed for the
Mouse (1:500, A11001, Invitrogen). All samples were field of tissue engineering [30,31] . HERS-DPCs constructs
observed under confocal microscope (Olympus FV1200, were printed with two kinds of bioinks - each layer of
Japan). HERS cells followed by two layers of DPCs. In other
words, the ratio of two cells is 1:2, which was the same as
2.4. Bioink preparation in our previous research . In this study, we used 27-gauge
[10]
The process of bioink preparation was performed syringe needle, and the related printing parameters
according to the manufacturer’s instructions. The first were set as follows: (i) pneumatic pressure: 0.8–1.1 bar;
step was to prepare the 0.25% (w/v) standard solution (ii) moving speed: 9–11 mm/s; (iii) temperature of print
of lithium phenyl-2, 4, 6-trimethylbenzoylphosphinate head: 25–27℃; (iv) temperature of platform: 15°C; and (v)
(LAP), which served as a photoinitiator. The LAP was distance between lines: 0.8 mm. Supplemented with culture
dissolved in phosphate buffered saline at 40–50℃ for medium after printing, the constructs were solidified with
15 min. Then lyophilized GelMA (GM-60, EFL, China) 405 nm light-curing portable source (EFL-LS-1601-405,
was dissolved in LAP solution at 37°C for 30 min and EFL, China) for 30 s [25,32] . To evaluate the function of
10% (w/v) GelMA solution was filtered through 0.22 μm HERS-DPCs construct in osteogenesis, HERS cells alone
sterile filter. At last, cells were suspended by GelMA construct and DPCs alone construct were bioprinted
142 International Journal of Bioprinting (2022)–Volume 8, Issue 3