Page 282 - IJB-8-3
P. 282
3D-bioprinted Ovary Initiated Puberty in the Model Mice
Figure 2. The preparation of ovary-derived decellularized extracellular matrix (dECM)-based bioink and the printing of porous cylindrical
3D scaffold constructs.
E
A
B D
C
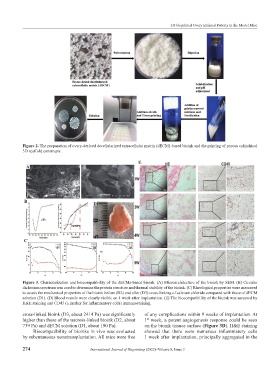
Figure 3. Characterization and biocompatibility of the dECMs-based bioink. (A) Microarchitecture of the bioink by SEM. (B) Circular
dichroism spectrum was used to determine the protein structure and thermal stability of the bioink. (C) Rheological properties were measured
to assess the mechanical properties of the bioink before (D2) and after (D3) cross-linking of calcium chloride compared with those of dECM
solution (D1). (D) Blood vessels were clearly visible on 1 week after implantation. (E) The biocompatibility of the bioink was assessed by
H&E staining and CD45 (a marker for inflammatory cells) immunostaining.
cross-linked bioink (D3, about 2414 Pa) was significantly of any complications within 9 weeks of implantation. At
higher than those of the uncross-linked bioink (D2, about 1 week, a potent angiogenesis response could be seen
st
739 Pa) and dECM solution (D1, about 190 Pa). on the bioink tissues surface (Figure 3D). H&E staining
Biocompatibility of bioinks in vivo was evaluated showed that there were numerous inflammatory cells
by subcutaneous xenotransplantation. All mice were free 1 week after implantation, principally aggregated in the
274 International Journal of Bioprinting (2022)–Volume 8, Issue 3