Page 155 - IJB-8-4
P. 155
Yang, et al.
A B C
D E F
G
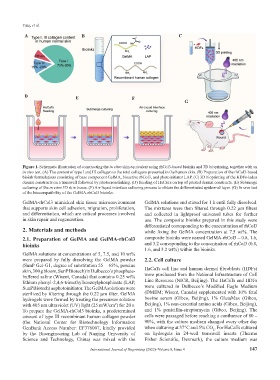
Figure 1. Schematic illustration of constructing the in vitro skin equivalent using rhCol3-based bioinks and 3D bioprinting, together with an
in vivo test. (A) The content of type I and III collagen to the total collagens presented in the human skin. (B) Preparation of the rhCol3-based
bioink formulations consisting of base component GelMA, bioactive rhCol3, and photoinitiator LAP. (C) 3D bioprinting of the HDFs-laden
dermal constructs on a transwell followed by photocrosslinking. (D) Seeding of HaCaTs on top of printed dermal constructs. (E) Submerge
culturing of the in vitro 3D skin tissue. (F) Air-liquid interface culturing process to obtain the differentiated epidermal layer. (G) In vivo test
of the biocompatibility of the GelMA-rhCol3 bioinks.
GelMA-rhCol3 mimicked skin tissue microenvironment GelMA solutions and stirred for 1 h until fully dissolved.
that supports skin cell adhesion, migration, proliferation, The mixtures were then filtered through 0.22 μm filters
and differentiation, which are critical processes involved and collected in lightproof universal tubes for further
in skin repair and regeneration. use. The composite bioinks prepared in this study were
differentiated corresponding to the concentration of rhCol3
2. Materials and methods while fixing the GelMA concentration at 7.5 wt%. The
2.1. Preparation of GelMA and GelMA-rhCol3 composite bioinks were named GelMA-rhCol3 – 0.8, 1.6,
bioinks and 3.2 corresponding to the concentration of rhCol3 (0.8,
1.6, and 3.2 wt%) within the bioinks.
GelMA solutions at concentrations of 5, 7.5, and 10 wt%
were prepared by fully dissolving the GelMA powder 2.2. Cell culture
(SunP Gel-G1, degree of substitution 55 – 65%, porcine
skin, 300 g bloom, SunP Biotech) in Dulbecco’s phosphate- HaCaTs cell line and human dermal fibroblasts (HDFs)
buffered saline (Wisent, Canada) that contains 0.25 wt% were purchased from the National Infrastructure of Cell
lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP, Line Resource (NICR, Beijing). The HaCaTs and HDFs
SunP Biotech) as photoinitiator. The GelMA solutions were were cultured in Dulbecco’s Modified Eagle Medium
sterilized by filtering through the 0.22 μm filter. GelMA (DMEM; Wisent, Canada) supplemented with 10% fetal
hydrogels were formed by treating the precursor solution bovine serum (Gibco, Beijing), 1% GlutaMax (Gibco,
with 405 nm ultraviolet (UV) light (25 mW/cm ) for 20 s. Beijing), 1% non-essential amino acids (Gibco, Beijing),
2
To prepare the GelMA-rhCol3 bioinks, a predetermined and 1% penicillin-streptomycin (Gibco, Beijing). The
amount of type III recombinant human collagen powder cells were passaged before reaching a confluence of 80 –
(the National Center for Biotechnology Information 90%, with the culture medium changed every other day
GenBank Access Number: EF376007, kindly provided when culturing at 37°C and 5% CO . For HaCaTs cultured
2
by the Bioengineering Lab of Nanjing University of on hydrogels in 24-well transwell inserts (Thermo
Science and Technology, China) was mixed with the Fisher Scientific, Denmark), the culture medium was
International Journal of Bioprinting (2022)–Volume 8, Issue 4 147