Page 159 - IJB-8-4
P. 159
Yang, et al.
A B C
D
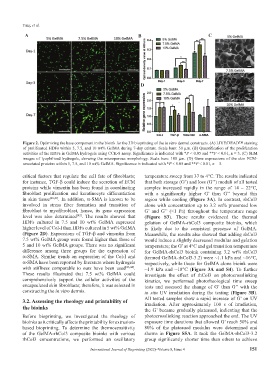
Figure 2. Optimizing the base component in the bioink for the 3D bioprinting of the in vitro dermal constructs. (A) LIVE/DEAD™ staining
of proliferated HDFs within 5, 7.5, and 10 wt% GelMA during 7-day culture. Scale bars: 50 μm. (B) Quantification of the proliferation
activities of the HDFs in GelMA hydrogels using CCK-8 assay. Significance is indicated with *P < 0.05 and **P < 0.01, n = 3. (C) SEM
images of lyophilized hydrogels, showing the microporous morphology. Scale bars: 100 μm. (D) Gene expressions of the skin ECM-
associated proteins within 5, 7.5, and 10 wt% GelMA. Significance is indicated with *P < 0.05 and **P < 0.01, n = 3.
critical factors that regulate the cell fate of fibroblasts; temperature sweep from 37 to 4°C. The results indicated
for instance, TGF-β could induce the secretion of ECM that both storage (G’) and loss (G’’) moduli of all tested
proteins while vimentin has been found in coordinating samples increased rapidly in the range of 14 – 22°C,
fibroblast proliferation and keratinocyte differentiation with a significantly higher G’ than G’’ beyond this
in skin tissue [40,41] . In addition, α-SMA is known to be region while cooling (Figure 3A). In contrast, rhCol3
involved in stress fiber formation and transition of alone with concentration up to 3.2 wt% presented low
fibroblast to myofibroblast, hence, its gene expression G’ and G’’ (<1 Pa) throughout the temperature range
level was also determined . The results showed that (Figure S3). These results evidenced the thermal
[42]
HDFs cultured in 7.5 and 10 wt% GelMA expressed gelation of GelMA-rhCol3 composite bioinks, which
higher level of Col-I than HDFs cultured in 5 wt% GelMA is likely due to the consistent presence of GelMA.
(Figure 2D). Expressions of TGF-β and vimentin from Meanwhile, the results also showed that adding rhCol3
7.5 wt% GelMA group were found higher than those of would induce a slightly decreased modulus and gelation
5 and 10 wt% GelMA groups. There was no significant temperature; the G’ at 4°C and gel transition temperature
difference among three groups for the expression of for GelMA-rhCol3 bioink containing 3.2 wt% rhCol3
α-SMA. Similar trends on expression of the Col-I and (termed GelMA-rhCol3-3.2) were ~1.1 kPa and ~16°C,
α-SMA have been reported by literature where hydrogels respectively, while those for GelMA alone bioink were
with stiffness comparable to ours have been used [43,44] . ~1.9 kPa and ~18°C (Figure 3A and S4). To further
These results illustrated that 7.5 wt% GelMA could investigate the effect of rhCol3 on photocrosslinking
comprehensively support the cellular activities of the kinetics, we performed photorheological time sweep
encapsulated skin fibroblasts; therefore, it was selected in tests and assessed the change of G’ than G’’ with the
constructing the in vitro dermis. in situ UV irradiation during the testing (Figure 3B).
3.2. Assessing the rheology and printability of All tested samples show a rapid increase of G’ on UV
irradiation. After approximately 100 s of irradiation,
the bioinks the G’ became gradually plateaued, indicating that the
Before bioprinting, we investigated the rheology of photocrosslinking reaction approached the end. The UV
bioinks as it critically affects the printability for extrusion- exposure time durations that allowed G’ reach 50% and
based bioprinting. To determine the thermosensitivity 80% of the plateaued modulus were determined and
of the GelMA-rhCol3 composite bioinks with various shown in Figure S5A. It took the GelMA-rhCol3-3.2
rhCol3 concentrations, we performed an oscillatory group significantly shorter time than others to achieve
International Journal of Bioprinting (2022)–Volume 8, Issue 4 151