Page 162 - IJB-8-4
P. 162
Recombinant Human Collagen for 3D Bioprinting of Skin Equivalent
A B
C D
E
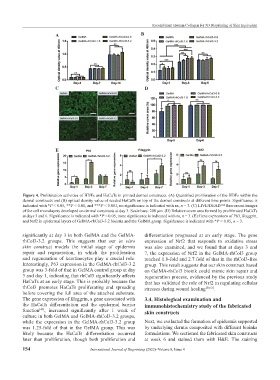
Figure 4. Proliferation activities of HDFs and HaCaTs in printed dermal constructs. (A) Quantified proliferation of the HDFs within the
dermal constructs and (B) optical density value of seeded HaCaTs on top of the dermal constructs at different time points. Significance is
indicated with *P < 0.05, **P < 0.01, and ***P < 0.001, no significance is indicated with ns, n = 3. (C) LIVE/DEAD™ fluorescent images
of the cell monolayers developed on dermal constructs at day 3. Scale bars: 200 μm. (D) Relative cover area formed by proliferated HaCaTs
at days 3 and 6. Significance is indicated with *P < 0.05, none significance is indicated with ns, n = 3. (E) Gene expressions of P63, filaggrin,
and Nrf2 in epidermal layers of GelMA-rhCol3-3.2 bioinks and the GelMA group. Significance is indicated with *P < 0.05, n = 3.
significantly at day 3 in both GelMA and the GelMA- differentiation progressed at an early stage. The gene
rhCol3-3.2 groups. This suggests that our in vitro expression of Nrf2 that responds to oxidative stress
skin construct models the initial stage of epidermis was also examined, and we found that at days 3 and
repair and regeneration, in which the proliferation 7, the expression of Nrf2 in the GelMA-rhCol3 group
and regeneration of keratinocytes play a crucial role. reached 1.8-fold and 2.7 fold of that in the rhCol3-free
Interestingly, P63 expression in the GelMA-rhCol3-3.2 group. This result suggests that our skin construct based
group was 3-fold of that in GelMA control group at day on GelMA-rhCol3 bioink could mimic skin repair and
3 and day 1, indicating that rhCol3 significantly affects regeneration process, evidenced by the previous study
HaCaTs at an early stage. This is probably because the that has validated the role of Nrf2 in regulating cellular
rhCol3 promotes HaCaTs proliferating and spreading stresses during wound healing [50,51] .
before covering the full area of the attached substrate.
The gene expression of filaggrin, a gene associated with 3.4. Histological examination and
the HaCaTs differentiation and the epidermal barrier immunohistochemistry study of the fabricated
function [49] , increased significantly after 1 week of skin constructs
culture in both GelMA and GelMA-rhCol3-3.2 groups,
while the expression in the GelMA-rhCol3-3.2 group Next, we evaluated the formation of epidermis supported
was 1.25-fold of that in the GelMA group. This was by underlying dermis composited with different bioinks
likely because the HaCaTs differentiation occurred formulations. We sectioned the fabricated skin constructs
later than proliferation, though both proliferation and at week 6 and stained them with H&E. The staining
154 International Journal of Bioprinting (2022)–Volume 8, Issue 4