Page 163 - IJB-8-4
P. 163
Yang, et al.
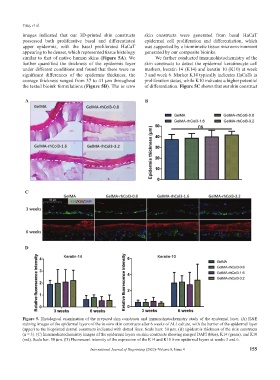
images indicated that our 3D-printed skin constructs skin constructs were generated from basal HaCaT
possessed both proliferative basal and differentiated epidermal cell proliferation and differentiation, which
upper epidermis, with the basal proliferated HaCaT was supported by a biomimetic tissue microenvironment
appearing to be denser, which represented tissue histology generated by our composite bioinks.
similar to that of native human skins (Figure 5A). We We further conducted immunohistochemistry of the
further quantified the thickness of the epidermis layer skin constructs to detect the epidermal keratinocyte cell
under different conditions and found that there were no markers, keratin 14 (K14) and keratin 10 (K10) at week
significant differences of the epidermis thickness, the 3 and week 6. Marker K14 typically indicates HaCaTs in
average thickness ranged from 37 to 41 μm throughout proliferation status, while K10 indicates a higher potential
the tested bioink formulations (Figure 5B). The in vitro of differentiation. Figure 5C shows that our skin construct
A B
C
D
Figure 5. Histological examination of the prepared skin constructs and immunohistochemistry study of the epidermal layer. (A) H&E
staining images of the epidermal layers of the in vitro skin constructs after 6 weeks of ALI culture, with the barrier of the epidermal layer
(upper) to the bioprinted dermal constructs indicated with dotted lines. Scale bars: 50 μm. (B) Epidermis thickness of the skin constructs
(n = 3). (C) Immunohistochemistry images of the epidermal layers on skin constructs showing merged DAPI (blue), K14 (green), and K10
(red). Scale bar: 50 μm. (D) Fluorescent intensity of the expression of the K14 and K10 from epidermal layers at weeks 3 and 6.
International Journal of Bioprinting (2022)–Volume 8, Issue 4 155