Page 309 - IJB-8-4
P. 309
Gu, et al.
A B C D E F
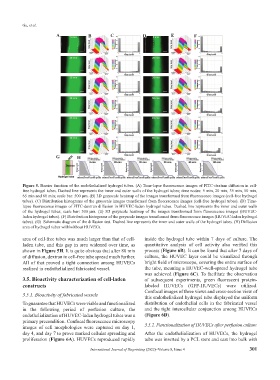
G H
Figure 5. Barrier function of the endothelialized hydrogel tubes. (A) Time-lapse fluorescence images of FITC-dextran diffusion in cell-
free hydrogel tubes. Dashed line represents the inner and outer walls of the hydrogel tubes; time nodes: 5 min, 20 min, 35 min, 50 min,
65 min and 80 min; scale bar: 500 μm. (B) 3D grayscale heatmap of the images transformed from fluorescence images (cell-free hydrogel
tubes). (C) Distribution histograms of the grayscale images transformed from fluorescence images (cell-free hydrogel tubes). (D) Time-
lapse fluorescence images of FITC-dextran diffusion in HUVEC-laden hydrogel tubes. Dashed line represents the inner and outer walls
of the hydrogel tubes; scale bar: 500 μm. (E) 3D grayscale heatmap of the images transformed from fluorescence images (HUVEC-
laden hydrogel tubes). (F) Distribution histograms of the grayscale images transformed from fluorescence images (HUVEC-laden hydrogel
tubes). (G) Schematic diagram of the diffusion test. Dashed line represents the inner and outer walls of the hydrogel tubes. (H) Diffusion
area of hydrogel tubes with/without HUVECs.
area of cell-free tubes was much larger than that of cell- inside the hydrogel tube within 7 days of culture. The
laden tube, and this gap in area widened over time, as quantitative analysis of cell activity also verified this
shown in Figure 5H. It is quite obvious that after 80 min process (Figure 6B). It can be found that after 7 days of
of diffusion, dextran in cell-free tube spread much further. culture, the HUVEC layer could be visualized through
All of that proved a tight connection among HUVECs bright field of microscope, covering the entire surface of
realized in endothelialized fabricated vessel. the tube, meaning a HUVEC-well-spread hydrogel tube
was achieved (Figure 6C). To facilitate the observation
3.5. Bioactivity characterization of cell-laden of subsequent experiments, green fluorescent protein-
constructs labeled HUVECs (GFP-HUVECs) were utilized.
Confocal images of three views and cross-section view of
3.5.1. Bioactivity of fabricated vessels this endothelialized hydrogel tube displayed the uniform
To guarantee that HUVECs were viable and functionalized distribution of endothelial cells in the fabricated vessel
in the following period of perfusion culture, the and the tight intercellular conjunction among HUVECs
endothelialization of HUVEC-laden hydrogel tubes was a (Figure 6D).
primary precondition. Confocal fluorescence microscopy
images of cell morphologies were captured on day 1, 3.5.2. Functionalization of HUVECs after perfusion culture
day 4, and day 7 to prove marked cellular spreading and After the endothelialization of HUVECs, the hydrogel
proliferation (Figure 6A). HUVECs reproduced rapidly tube was inserted by a PCL stent and cast into bulk with
International Journal of Bioprinting (2022)–Volume 8, Issue 4 301