Page 247 - IJB-9-1
P. 247
International Journal of Bioprinting 3D printing of smart constructs for precise medicine
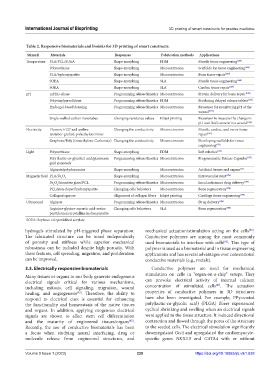
Table 2. Responsive biomaterials and bioinks for 3D printing of smart constructs.
Stimuli Materials Responses Fabrication methods Applications
Temperature PLA/PCL/SOEA Shape-morphing FDM Muscle tissue engineering [165]
Polyurethane Shape-morphing Microextrusion Scaffolds for tissue engineering [166]
PLA/hydroxyapatite Shape-morphing Microextrusion Bone tissue repair [167]
SOEA Shape-morphing SLA Muscle tissue engineering [168]
SOEA Shape-morphing SLA Cardiac tissue repair [169]
pH mPEG-silane Programming release kinetics Microextrusion Protein delivery for bone repair [170]
Polyvinylpyrrolidone Programming release kinetics FDM Producing delayed release tablets [171]
Hydrogel-based dressing Programming release kinetics Microextrusion Biosensor for monitoring pH of the
wound [172]
Single-walled carbon nanotubes Changing resistance values Inkjet printing Biosensor to measure the change in
pH and fluid content in a wound [144]
Electricity Pluronic F127 and aniline Changing the conductivity Microextrusion Muscle, cardiac, and nerve tissue
tetramer-grafted-polyethyleneimine repair [147]
Graphene/Poly (trimethylene Carbonate) Changing the conductivity Microextrusion Developing scaffolds for tissue
engineering [173]
Light Polyurethane Shape-morphing FDM Soft robotics [174]
Poly (lactic-co-glycolic) acid/plasmonic Programming release kinetics Microextrusion Programmable Release Capsules [175]
gold nanorods
Alginate/polydopamine Shape-morphing Microextrusion Artificial tissues and organs [13]
Magnetic field PLA/Fe O 4 Shape-morphing Microextrusion Intravascular stent [176]
3
Fe O /bioactive glass/PCL Programming release kinetics Microextrusion Local anticancer drug delivery [177]
3 4
PCL/iron-doped hydroxyapatite Changing cells behaviors Microextrusion Bone regeneration [178]
Collagen/agarose Alignment of collagen fibers Inkjet printing Cartilage tissue engineering [179]
Ultrasound Alginate Programming release kinetics Microextrusion Drug delivery [106]
Arginine-glycine-aspartic acid-serine Changing cells behaviors SLA Bone regeneration [180]
peptide/nanocrystalline hydroxyapatite
SOEA: Soybean oil epoxidized acrylate
hydrogels stimulated by pH-triggered phase separation. mechanical actuation/stimulation acting on the cells .
[83]
The fabricated structure can be tuned independently Conductive polymers are among the most commonly
of porosity and stiffness while superior mechanical used biomaterials to interface with cells . This type of
[83]
robustness can be included despite high porosity. With polymer is used as a biomaterial and in tissue engineering
these features, cell spreading, migration, and proliferation applications and has several advantages over conventional
can be improved. conductive materials (e.g., metals).
3.3. Electrically responsive biomaterials Conductive polymers are used for mechanical
Many tissues or organs in our body generate endogenous stimulation on cells in “organ-on-a-chip” setups. They
electrical signals critical for various mechanisms, can provoke electrical activity of internal calcium
[84]
including mitosis, cell signaling, migration, wound concentration of stimulated cells . The actuation
healing, and angiogenesis . Therefore, the ability to properties of conductive polymers in 3D structures
[82]
respond to electrical cues is essential for enhancing have also been investigated. For example, PPy-coated
the functionality and homeostasis of the native tissues poly(lactic-co-glycolic acid) (PLGA) fibers experienced
and organs. In addition, applying exogenous electrical cyclical shrinking and swelling when an electrical signals
signals are shown to affect stem cell differentiation were applied to the tissue structure. It induced directional
and the maturity of engineered tissues/organs . contraction and flowed through the pores of the structure
[82]
Recently, the use of conductive biomaterials has been to the seeded cells. The electrical stimulation significantly
a focus when studying neural interfacing, drug or downregulated Oct4 and upregulated the cardiomyocyte-
molecule release from engineered structures, and specific genes NKX2.5 and GATA4 with or without
Volume 9 Issue 1 (2023) 239 https://doi.org/10.18063/ijb.v9i1.638