Page 331 - IJB-9-2
P. 331
International Journal of Bioprinting Engineered EVs increase viability of 3D printed cardiomyocytes
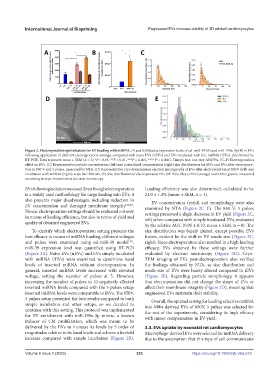
Figure 2. Electroporation optimization for EV loading with miRNA. (A and B) Relative expression levels of cel-miR-39 (A) and miR-199a-3p (B) in EVs
following application of different electroporation settings, compared with naïve EVs (nEVs) and EVs incubated with free miRNA (iEVs), determined by
RT-PCR. Data represent mean ± SEM (n = 3) *P < 0.05, **P < 0.01, ***P < 0.001, **** P < 0.0001; Tukey’s test, one-way ANOVA. (C–F) Electroporation
effect on EVs. (C) Representative particle concentration (left) and normalized concentration (right) size distributions for iEVs and EVs after electropora-
tion in 850 V and 5 pulses, measured by NTA. (D) Representative cryo-transmission electron micrographs of EVs after electroporation at 850V (left) and
incubation with miRNA (right); scale bar: 200 nm. (E) Size distribution of electroporated EVs (EP-EVs, blue), iEVs (orange) and nEVs (green), measured
according to cryo-transmission electron microscopy.
EVs following isolation was used. Even though electroporation Loading efficiency was also determined, calculated to be
is a widely used methodology for cargo loading into EVs, it 21.0 ± 1.3% (mean ± SEM, n = 4).
also presents major disadvantages, including reduction in EV concentration (yield) and morphology were also
EV concentration and damaged membrane integrity [44,45] . examined by NTA (Figure 2C–E). The 850 V, 5 pulses
Hence, electroporation settings should be evaluated not only settings presented a slight decrease in EV yield (Figure 2C,
in means of loading efficiency, but also in terms of yield and left) when compared with simply incubated EVs, evaluated
quality of obtained engineered EVs.
by the relative AUC (0.95 ± 0.10, mean ± SEM, n = 8). The
To identify which electroporation setting presents the size distribution was barely altered, except possible EVs
best efficacy in means of miRNA loading, different voltages fusion, evident by the shift in EV mode size (Figure 2C,
and pulses were examined using cel-miR-39 model . right). Since electroporation also resulted in a high loading
[46]
miR-39 expression level was quantified using RT-PCR efficacy, EVs obtained by these settings were further
(Figure 2A). Naïve EVs (nEVs) and EVs simply incubated evaluated by electron microscopy (Figure 2D). Cryo-
with miRNA (iEVs) were examined to determine basal TEM imaging of EVs post-electroporation also verified
levels of inserted miRNA without electroporation. In the findings obtained by NTA, as size distribution and
general, inserted miRNA levels increased with elevated mode size of EVs were barely altered compared to iEVs
voltage, setting the number of pulses at 5. However, (Figure 2E). Regarding particle morphology, it appears
increasing the number of pulses to 10 negatively affected that electroporation did not change the shape of EVs or
inserted miRNA levels compared with the 5 pulses setup; affect their membrane integrity (Figure 2D), meaning that
inserted miRNA levels were comparable to iEVs. The 850V, engineered EVs maintain their stability.
5 pulses setup presented the best results compared to both Overall, the optimal setting for loading selective miRNA
simple incubation and other setups, so we decided to into MΦs-derived EVs of 850V, 5 pulses was selected for
continue with this setting. This protocol was implemented the rest of the experiments, considering its high efficacy
for EV enrichment with miR-199a-3p mimic, a known with minor compensation in EV yield.
inducer of CM proliferation, which was meant to be
delivered by the EVs to increase its levels by 5 order of 3.3. EVs uptake by neonatal rat cardiomyocytes
magnitudes relative to its basal levels and achieve a fivefold Macrophage-derived EVs were selected for miRNA delivery
increase compared with simple incubation (Figure 2B). due to the assumption that this type of cell communicates
Volume 9 Issue 2 (2023) 323 https://doi.org/10.18063/ijb.v9i2.670