Page 332 - IJB-9-2
P. 332
International Journal of Bioprinting Engineered EVs increase viability of 3D printed cardiomyocytes
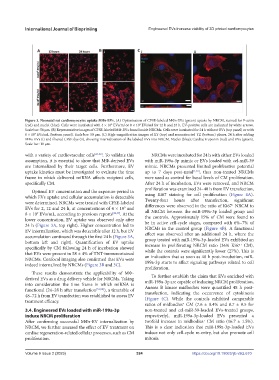
Figure 3. Neonatal rat cardiomyocytes uptake MΦs-EVs. (A) Optimization of CFSE-labeled MΦs-EVs (green) uptake by NRCM, stained for F-actin
(red) and nuclei (blue). Cells were incubated with 4 × 10 EVs/ml or 8 × 10 EVs/ml for 12 h and 24 h. EV-positive cells are indicated by white arrows.
9
9
Scale bar: 50 µm. (B) Representative images of CFSE-labeled MΦ-EVs found inside NRCMs. Cells were incubated for 24 h without EVs (top panel) or with
6 × 10 EVs/mL (bottom panel). Scale bar: 50 µm. (C) High-magnification images of XY (top) and reconstructed YZ (bottom) planes, 24 h after adding
9
MΦs-EVs (i) and filtered CFSE dye (ii), showing internalization of the labeled EVs into NRCM. Nuclei (blue); Cardiac troponin (red) and EVs (green).
Scale bar: 10 µm.
with a variety of cardiovascular cells [47-50] . To validate this NRCMs were incubated for 24 h with either EVs loaded
assumption, it is essential to show that MΦ-derived EVs with miR-199a-3p mimic or EVs loaded with cel-miR-39
are internalized by their target cells. Furthermore, EV mimic. NRCMs presented limited proliferative potential
uptake kinetics must be investigated to evaluate the time up to 7 days post-natal [1,53] , thus non-treated NRCMs
frame in which delivered miRNA affects recipient cells, were used as control for basal levels of CM proliferation.
specifically CM. After 24 h of incubation, EVs were removed, and NRCM
Optimal EV concentration and the exposure period in proliferation was examined 24–48 h from EV transfection,
which EVs uptake and cellular accumulation is detectable using Ki67 staining for cell proliferation (Figure 4A).
were determined. NRCMs were treated with CFSE-labeled Twenty-four hours after transfection, + significant
EVs for 2, 12 and 24 h, at concentrations of 4 × 10 and differences were observed in the ratio of Ki67 NRCM to
9
8 × 10 EVs/mL, according to previous reports [22,37] . At the all NRCM between the miR-199a-3p loaded group and
9
lower concentration, EV uptake was observed only after the controls. Approximately 15% of CM were found to
24 h (Figure 3A, top right). Higher concentration led to be in active cell-cycle stages, compared with 6%–7% of
EV internalization, which was detectable after 12 h, but EV NRCMs in the control group (Figure 4B). A functional
accumulation continued through the first 24 h (Figure 3A, effect was observed after an additional 24 h, where the
bottom left and right). Quantification of EV uptake group treated with miR-199a-3p-loaded EVs exhibited an
+
specifically by CM following 24 h of incubation showed increase in proliferating NRCM ratio (36% Ki67 CM),
that EVs were present in 58 ± 4% of TNT-immunostained while the controls were significantly lower (27%). This is
NRCMs. Confocal imaging also confirmed that EVs were an indication that as soon as 48 h post-incubation, miR-
indeed internalized by NRCMs (Figure 3B and 3C). 199a-3p starts to affect signaling pathways related to cell
proliferation.
These results demonstrate the applicability of MΦ-
To further establish the claim that EVs enriched with
derived EVs as a drug delivery vehicle for NRCMs. Taking miR-199a-3p are capable of inducing NRCM proliferation,
into consideration the time frame in which miRNA is Aurora B kinase midbodies were quantified 48 h post-
functional (24–28 h after transfection [51,52] ), a timetable of transfection, indicating the occurrence of cytokinesis
48–72 h from EV transfection was established to assess EV (Figure 4C). While the controls exhibited comparable
treatment efficacy.
ratios of midbodies CM (7.6 ± 0.4% and 8.7 ± 0.5 for
+
3.4. Engineered EVs loaded with miR-199a-3p non-treated and cel-miR-39-loaded EVs-treated groups,
induce NRCM proliferation respectively), miR-199a-3p-loaded EVs presented a
After confirming successful MΦs-EV internalization by twofold increase in midbodies CM ratio (16.7 ± 1.5%).
+
NRCM, we further assessed the effect of EV treatment on This is a clear indication that miR-199a-3p-loaded EVs
cardiac regeneration-related cellular processes, such as CM induce not only cell-cycle re-entry, but also promote cell
proliferation. mitosis.
Volume 9 Issue 2 (2023) 324 https://doi.org/10.18063/ijb.v9i2.670