Page 333 - IJB-9-2
P. 333
International Journal of Bioprinting Engineered EVs increase viability of 3D printed cardiomyocytes
+
+
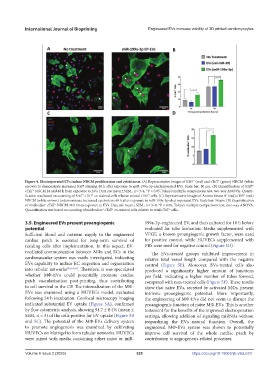
Figure 4. Electroporated EVs induce NRCM proliferation and cytokinesis. (A) Representative images of Ki67 (red) and cTnT (green) NRCM (white
arrows) to demonstrate increased Ki67 staining 48 h after exposure to miR-199a-3p-electroporated EVs. Scale bar: 50 μm. (B) Quantification of Ki67 +
+
cTnT NRCM 24 and 48 h from exposure to EVs. Data are mean ± SEM., n = 3–4, *P < 0.05, Tukey’s multiple comparisons test, two-way ANOVA. Quanti-
+
+
+
+
fication was based on counting of Ki67 cTnT co-stained cells relative to total cTnT cells. (C) Representative images of Aurora kinase B (red) cTnT (red)
+
NRCM (white arrows) to demonstrate increased cytokinesis 48 h after exposure to miR-199a-3p-electroporated EVs. Scale bar: 50 μm. (B) Quantification
of midbodies cTnT NRCM 48 h from exposure to EVs. Data are mean ± SEM., n = 3–4, *P < 0.05, Tukey’s multiple comparisons test, one-way ANOVA.
+
+
+
Quantification was based on counting of midbodies cTnT co-stained cells relative to total cTnT cells.
+
+
3.5. Engineered EVs present proangiogenic 199a-3p-engineered EV, and then cultured for 18 h before
potential evaluated for tube formation. Media supplemented with
Sufficient blood and nutrient supply to the engineered VEGF, a known proangiogenic growth factor, were used
cardiac patch is essential for long-term survival of for positive control, while HUVECs supplemented with
residing cells after implementation. In this aspect, EV- PBS were used for negative control (Figure 5D).
mediated communication between MΦs and ECs in the The EVs-treated groups exhibited improvement in
cardiovascular system was vastly investigated, indicating relative total vessel length compared with the negative
EVs capability to induce EC migration and organization control (Figure 5E). Moreover, EVs-treated cells also
into tubular networks [35,54,55] . Therefore, it was speculated produced a significantly higher amount of junctions
whether MΦ-EVs could potentially promote cardiac per field, indicating a higher number of tubes formed,
patch vascularization post-printing, thus contributing compared with non-treated cells (Figure 5F). These results
to cell survival in the CP. The internalization of the MΦ- show that naïve EVs, secreted by activated MΦs, present
EVs was examined using a HUVECs model, evaluated intrinsic proangiogenic potential. More importantly,
following 24 h incubation. Confocal microscopy imaging the engineering of MΦ-EVs did not seem to disrupt the
indicated substantial EV uptake (Figure 5A), confirmed proangiogenic function of naïve MΦ-EVs. This is another
by flow cytometric analysis, showing 94.7 ± 0.4% (mean ± testament for the benefits of the improved electroporation
SEM, n = 3) of the cells positive for EV uptake (Figure 5B settings, allowing addition of signaling miRNAs without
and 5C). The potential of the MΦ-EVs delivery system jeopardizing the EVs natural function. Overall, the
to promote angiogenesis was examined by cultivating engineered MΦ-EVs system was shown to potentially
HUVECs on Matrigel to form tubular networks. HUVECs improve cell survival of the whole cardiac patch by
were mixed with media containing either naïve or miR- contribution to angiogenesis-related processes.
Volume 9 Issue 2 (2023) 325 https://doi.org/10.18063/ijb.v9i2.670