Page 335 - IJB-9-2
P. 335
International Journal of Bioprinting Engineered EVs increase viability of 3D printed cardiomyocytes
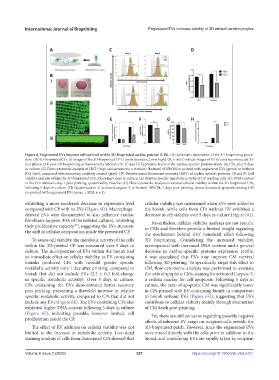
Figure 6. Engineered EVs improve cell survival within 3D-bioprinted cardiac patches (CPs). (A) Schematic description of the 3D bioprinting proce-
dure. (B) 3D-bioprinted CPs. (i) Images of the 3D-bioprinted CP (1 cm in diameter, 2 mm high). (B, ii–iii) Confocal images of XY (ii) and reconstructed YZ
(iii) planes, 24 h post-3D bioprinting of fluorescently labeled cells. (C and D) Expression levels of the cardiac-specific proteins inside the CPs, after 5 days
in culture. (C) Flow cytometric analysis of cTnT (top) and sarcomeric α-Actinin (bottom) of NRCM co-printed with engineered EVs (green) or without
+
+
EVs (red), compared with secondary antibody control (gray). (D) Relative mean fluorescent intensity (MFI) of cardiac-specific proteins. (E and F) Cell
viability analysis within the 3D-bioprinted CPs, following 5 days in culture. (E) Relative specific metabolic activity of CP residing cells. (F) DNA content
in the CPs relative to day 1 post-printing, quantified by Hoechst. (G) Flow cytometric analysis of relative cellular viability within the 3D-bioprinted CPs,
following 5 days in culture. (H) Quantification of activated caspase 3 α-Actinin NRCM, 5 days post-printing, shows decreased apoptosis among CM
+
+
co-printed with engineered EVs (mean ± SEM, n = 3).
exhibiting a more moderate decrease in expression level cellular viability was maintained when EVs were added to
compared with CP with no EVs (Figure 6D). Macrophage- the bioink, while cells from CPs without EV exhibited a
derived EVs were documented to also influence cardiac decrease in cell viability over 5 days in culture (Figure 6G).
fibroblasts (approx. 40% of the isolated culture), inhibiting Nevertheless, cellular viability analyses are not specific
their proliferative capacity , suggesting the EVs attenuate to CMs and therefore provide a limited insight regarding
[57]
the shift in cellular composition inside the presented CP. the mechanism behind EVs’ beneficial effect following
To assess cell viability, the metabolic activity of the cells 3D bioprinting. Considering the increased viability,
within the 3D-printed CP was measured over 5 days in accompanied with increased DNA content and a general
culture. The incorporation of EVs within the bioink had decrease in cardiac-specific proteins expression profile,
an immediate effect on cellular viability, as EV-containing it was speculated that EVs may improve CM survival
bioinks produced CPs with twofold greater specific following 3D printing. To specifically target this effect in
metabolic activity only 1 day after printing, compared to CM, flow-cytometric analysis was performed to evaluate
bioink that did not include EVs (2.3 ± 0.2 fold-change the ratio of apoptotic CMs, staining for activated Caspase-3,
in specific metabolic activity). Over 5 days in culture, a cellular marker for cell apoptosis. Following 5 days in
CPs containing the EVs demonstrated better recovery culture, the ratio of apoptotic CM was significantly lower
post-printing, presenting a threefold increase in relative in CPs printed with EV-containing bioink in comparison
specific metabolic activity, compared to CPs that did not to bioink without EVs (Figure 6H), suggesting that EVs
include any EVs (Figure 6E). The EVs-containing CPs also contribute to cellular viability mainly through attenuation
exhibited higher DNA content following 5 days in culture of CM death post-printing.
(Figure 6F), indicating possible, however limited, cell Yet, there are still concerns regarding possible negative
proliferation inside the CP.
effects of inherent EV cargo on recipient cells outside the
The effect of EV addition on cellular viability was not 3D-bioprinted patch. However, since the engineered EVs
limited to the increase in metabolic activity. Live–dead were mixed directly with the cells prior to addition to the
staining analysis of cells from dissociated CPs showed that bioink and considering EVs are rapidly taken by recipient
Volume 9 Issue 2 (2023) 327 https://doi.org/10.18063/ijb.v9i2.670