Page 334 - IJB-9-2
P. 334
International Journal of Bioprinting Engineered EVs increase viability of 3D printed cardiomyocytes
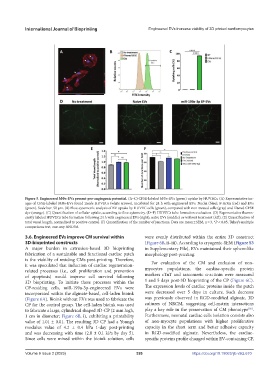
Figure 5. Engineered MΦs-EVs present pro-angiogenic potential. (A–C) CFSE-labeled MΦs-EVs (green) uptake by HUVECs. (A) Representative im-
ages of CFSE-labeled MΦs-EVs found inside HUVECs (white arrows), incubated for 24 h with engineered EVs. Nuclei (blue); F-Actin (red) and EVs
(green). Scale bar: 50 µm. (B) Flow cytometric analysis of EV uptake by HUVEC cells (green), compared with non-treated cells (gray) and filtered CFSE
dye (orange). (C) Quantification of cellular uptake, according to flow cytometry. (D–F) HUVECs tube formation evaluation. (D) Representative fluores-
cently labeled HUVECs tube formation following 24 h with engineered EVs (right), naïve EVs (middle) or without treatment (left). (E) Quantification of
total vessel length, normalized to positive control. (F) Quantification of the number of junctions. Data are mean ± SEM, n = 3, *P < 0.05, Tukey’s multiple
comparisons test, one-way ANOVA.
3.6. Engineered EVs improve CM survival within were evenly distributed within the entire 3D construct
3D-bioprinted constructs (Figure 6B, ii–iii). According to cryogenic-SEM (Figure S3
A major burden in extrusion-based 3D bioprinting in Supplementary File), EVs maintained their sphere-like
fabrication of a sustainable and functional cardiac patch morphology post-printing.
is the viability of residing CMs post-printing. Therefore,
it was speculated that induction of cardiac regeneration- For evaluation of the CM and exclusion of non-
related processes (i.e., cell proliferation and prevention myocytes populations, the cardiac-specific protein
of apoptosis) would improve cell survival following markers cTnT and sarcomeric α-actinin were measured
3D bioprinting. To initiate these processes within the 1 and 5 days post-3D bioprinting of the CP (Figure 6C).
CP-residing cells, miR-199a-3p-engineered EVs were The expression levels of cardiac proteins inside the patch
incorporated within the alginate-based, cell-laden bioink were decreased over 5 days in culture. Such decrease
(Figure 6A). Bioink without EVs was used to fabricate the was previously observed in RGD-modified alginate, 3D
CP for the control group. The cell-laden bioink was used cultures of NRCM, suggesting cell-matrix interactions
[56]
to fabricate a large, cylindrical shaped 3D-CP (2 mm high, play a key role in the preservation of CM phenotype .
1 cm in diameter; Figure 6B, i), exhibiting a printability Furthermore, neonatal cardiac cells isolation consists also
value of 1.01 ± 0.01. The resulting 3D CP had a Young’s of non-myocyte populations with higher proliferative
modulus value of 4.2 ± 0.4 kPa 1-day post-printing capacity in the short term and better adhesive capacity
and was decreasing with time (2.0 ± 0.1 kPa by day 5). in RGD-modified alginate. Nevertheless, the cardiac-
Since cells were mixed within the bioink solution, cells specific proteins profile changed within EV-containing CP,
Volume 9 Issue 2 (2023) 326 https://doi.org/10.18063/ijb.v9i2.670