Page 406 - IJB-9-2
P. 406
International Journal of Bioprinting 3D-printed skin substitute accelerates wound healing in vivo
3.2. Preparation of dECM-GelMA-HAMA precursor 3.2.3. SEM imaging
3.2.1. DAPI staining and DNA quantitative analysis of The interior structure of this photo-crosslinked dECM-
adipose tissue dECM GelMA-HAMA composite hydrogel was a high porous
No blue fluorescent nuclear components were observed 3D network structure, and uniform pore distribution was
in DAPI staining of lyophilized adipose tissue dECM observed inside the composite hydrogel. (Figure 3A) The
(Figure 1A). DNA quantitative analysis showed that porosity was 65% and the average pore size was 73 ± 18 μm,
adipose tissue dECM obtained in section 2.2.1 contained which is conducive for the survival, accumulation and
negligible amounts of DNA (24.5 ± 7.1 ng/mg), meeting proliferation of encapsulated cells and is suitable for tissue
the currently accepted decellularization criteria, and the engineering application.
residual DNA was <50 ng/mg . Adipose tissue dECM pre- 3.3. 3D-bioprinted dECM-GelMA-HAMA skin
[9]
gel is a thermo-sensitive biomaterial. At low temperatures substitute loaded with hADSCs
(0 – 4°C), the adipose tissue dECM pre-gel was free-
flowing and gelated when temperature rises to 37°C A cylindrical scaffold loaded with hADSCs with the
gradually, which can resist its own gravity after gelation diameter of 8 mm was printed by an extrusion-based
[10]
(Figure 1B and C). 3D printer. The dECM-GelMA-HAMA skin substitute
was transparent and soft, with stereoscopic grid-like
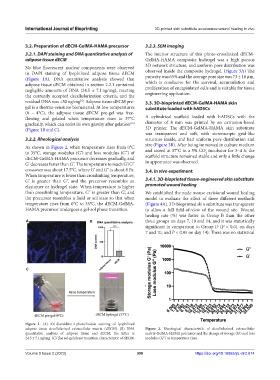
3.2.2. Rheological analysis structure inside, and had uniform pore distribution and
As shown in Figure 2, when temperature rises from 0°C size (Figure 3B). After being immersed in culture medium
to 35°C, storage modulus (G’) and loss modulus (G’’) of and stored at 37°C in a 5% CO incubator for 3–4 h, the
2
dECM-GelMA-HAMA precursor decreases gradually, and scaffold structure remained stable and only a little change
G’ decreases faster than G’’. The temperature to reach G’/G’’ in appearance was observed.
crossover was about 17.5°C, where G’ and G’’ is about 8 Pa. 3.4. In vivo experiment
When temperature is lower than crosslinking temperature,
G’ is greater than G’’, and the precursor resembles an 3.4.1. 3D-bioprinted tissue-engineered skin substitute
elastomer or hydrogel state. When temperature is higher promoted wound healing
than crosslinking temperature, G’’ is greater than G’, and We established the nude mouse excisional wound healing
the precursor resembles a fluid or sol state so that when model to evaluate the effect of three different methods
temperature rises from 0°C to 35°C, the dECM-GelMA- (Figure 4A). 3D-bioprinted skin substitute was transparent
HAMA precursor undergoes a gel-sol phase transition. to allow a full field-of-view of the wound site. Wound
healing rate (%) was faster in Group B than the other
A B three groups on days 7, 10 and 14, and it was statistically
significant in comparison to Group D (P < 0.01 on days
7 and 10, and P < 0.05 on day 14). There was no statistical
C
Figure 1. (A) 4’,6-diamidino-2-phenylindole staining of lyophilized
adipose tissue decellularized extracellular matrix (dECM). (B) DNA Figure 2. Rheological characteristic of decellularized extracellular
quantitative analysis of adipose tissue and dECM, the latter is matrix-GelMA-HAMA precursor and the change of storage (G’) and loss
24.5 ± 7.1 ng/mg. (C) The sol-gel phase transition characteristic of dECM. modulus (G’’) as temperature rises.
Volume 9 Issue 2 (2023) 398 https://doi.org/10.18063/ijb.v9i2.674